Critical Care
The Southwest Journal of Pulmonary and Critical Care publishes articles directed to those who treat patients in the ICU, CCU and SICU including chest physicians, surgeons, pediatricians, pharmacists/pharmacologists, anesthesiologists, critical care nurses, and other healthcare professionals. Manuscripts may be either basic or clinical original investigations or review articles. Potential authors of review articles are encouraged to contact the editors before submission, however, unsolicited review articles will be considered.
ACE Inhibitor Related Angioedema: A Case Report and Brief Review
F. Brian Boudi, J. L. Rush, Cameron Farsar, Connie S. Chan
Carl T. Hayden VA Medical Center
University of Arizona, College of Medicine Phoenix Campus
Phoenix, AZ USA
Abstract
We present a case report of angiotensin converting enzyme (ACE) inhibitor angioedema successfully treated with icatibant (Firazyr®). The pathophysiology and treatment of ACE inhibitor angioedema is reviewed.
Introduction
Angioedema, swelling caused by a rapid increase in permeability of submucosal or subcutaneous capillaries and post-capillary venules with localized plasma extravasation, is associated with random, highly variable and often unpredictable clinical manifestations (1). Attacks are associated with significant decreased quality of life both during and between attacks, significant functional impairment and a high risk of morbidity and mortality. Angioedema can be caused by either mast cell degranulation or activation of the kallikrein-kinin cascade. ACE inhibitor-related angioedema is one the leading causes of drug-induced angioedema. While ACE inhibitor-induced angioedema is rare, awareness of this serious and potentially life-threatening complication is of great importance because of the extensive use of this class of drugs in clinical practice. Cases presenting into the emergency department because ACE inhibitors, one of the most widely prescribed medications prescribed in the United States, account for about 20-40 percent of emergency room admissions related to angioedema (1,2).
Approximately 50% of patients with ACE inhibitor-induced angioedema arise within the first week of treatment. The remainder can become symptomatic weeks, months, or even years later. The estimated incidence is likely underestimated. The actual incidence can be far higher because of poorly recognized presentation of angioedema and its sometimes-late onset. The incidence can be even higher (up to 3-fold) in certain risk groups, for instance Afro-Americans (3). It seems to have a predilection for the head, neck, lips, mouth, tongue, larynx, pharynx, and subglottal areas without urticaria (4).
Case Presentation
A 55-year-old veteran presented to the Emergency Department for the Carl T. Hayden Veterans Administration Medical Center in Phoenix Arizona with impressive angioedema. The Veteran had been taking lisinopril for 6 years and had another similar episode two months prior. The prior episode presented with facial swelling that resolved within a couple of hours. However, the present episode was accompanied by difficulty breathing and swallowing. He was begun on an allergic reaction protocol which included establishing and making sure the veteran had a patent airway, nasal trumpet, placing a peripheral intravenous catheter and starting iv fluid of sodium chloride 0.9% to keep vein open, medications of diphenhydramine 50 mg, famotidine 20 mg, methylprednisolone 125mg and 0.3 mg epinephrine subcutaneously. He was also given racemic epinephrine mixed via nebulizer and 30 mg subcutaneously of icatibant (Firazyr®), a bradykinin B2 receptor antagonist used to treat hereditary angioedema. He improved and was subsequently admitted to the intensive care unit for continued observation. The following day he was discharged with prescriptions for prednisone and orders to discontinue the use of lisinopril.
Discussion
Despite newer therapies, there are no currently approved guidelines for the treatment of ACE inhibitor-induced angioedema in the United States. It is difficult to tell whether icatibant was truly effective in this case presentation as it was one of multiple therapies administered. Many causes of angioedema result from release of histamine (1). However, ACE inhibitor angioedema results from other inflammatory mediators, especially bradykinin (2) (Figure 1).

Figure 1. Simplified pathway for bradykinin-mediated angioedema showing the sites of drug activity (5).
Mast cells are not believed to be involved in this form of angioedema, and pruritus and urticaria are absent. Bradykinin-mediated angioedema, unlike histamine-mediated angioedema, frequently affects the gastrointestinal mucosa, leading to bowel wall edema and presenting with episodes of abdominal pain, nausea, vomiting, and/or diarrhea. While antihistamines and corticosteroids are often administered for treatment of angioedema, they are unlikely to have effect in ACE inhibitor induced angioedema. Epinephrine may slow (or stop) the rate of swelling. ACE inhibitor angioedema may be treated with additional drugs that act on the bradykinin pathway (e.g., icatibant, ecallantide). The recommended dose of icatibant is 30 mg administered by subcutaneous (SC) injection in the abdominal area. Additional doses may be administered in 6 hours if response is inadequate. Icatibant may decrease the time of recovery from ACE inhibitor related angioedema (6). Another ACE inhibitor should not be prescribed as the reaction is a class, not a drug specific reaction (7). Checking the complement C4 may be helpful. Patients with preexisting angioedema, including hereditary angioedema caused by C1 esterase inhibitor deficiency, are predisposed to develop angioedema in response to ACE inhibitors (8).
ACE inhibitor induced angioedema remains a disorder without a clear treatment modality for reduction of symptoms. The primary therapeutic interventions remain removal of the offending agent and airway management when indicated. The use of icatibant may be effective in the management of ACE inhibitor related angioedema; however, its efficacy and benefits have not been clear in the small studies published thus far. There have been three randomized trials evaluating the use of icatibant in ACE inhibitor angioedema. Interestingly, the first study found icatibant to be effective while the more recent and larger studies found no significant difference in time to recovery (3, 6, 9-12). Icatibant is costly with a wholesale price of $9,000-$11,000 and may not be available at all hospitals. Given its questionable outcomes data, icatibant may not appropriate in all medical centers. This is especially important since off-label use may not be covered by insurers.
References
- Stone C Jr, Brown NJ. Angiotensin-converting enzyme inhibitor and other drug-associated angioedema. Immunol Allergy Clin North Am. 2017 Aug;37(3):483-495. [CrossRef] [PubMed]
- Guyer AC, Banerji A. ACE inhibitor-induced angioedema. UpToDate. June 27, 2017. Available at: https://www.uptodate.com/contents/an-overview-of-angioedema-clinical-features-diagnosis-and-management#H30 (requires subscription, accessed 9/18/17).
- Straka BT, Ramirez CE, Byrd JB, et al. Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J Allergy Clin Immunol. 2017;140:242-248.e2. [CrossRef] [PubMed]
- Sabroe R, Black A. Angiotensin-converting enzyme (ACE) inhibitors and angio-oedema. Br J Dermatol. 1997;1:153–8. [CrossRef] [PubMed]
- Shenvi C, Serrano K. New treatments for angioedema. Emergency Physicians Monthly. 9/12/16. Available at: http://epmonthly.com/article/new-treatments-angioedema/ (accessed 10/20/17).
- Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015 Jan 29;372(5):418-25. [CrossRef] [PubMed]
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT.Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med. 2005;1:1428-9. [CrossRef] [PubMed]
- Orfan N, Patterson R, Dykewicz M. Severe angioedema related to ACE inhibitors in patients with a history of idiopathic angioedema. JAMA. 1990;1:1287-9. [CrossRef] [PubMed]
- Sinert R, Levy P, Bernstein JA, et al.Randomized trial of icatibant for angiotensin-converting enzyme inhibitor-induced upper airway angioedema. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1402-9.e3. [CrossRef] [PubMed]
- Culley CM, DiBridge JN, Wilson GL Jr. Off-label use of agents for management of serious or life-threatening angiotensin converting enzyme inhibitor-induced angioedema. Ann Pharmacother. 2016 Jan;50(1):47-59 [CrossRef] [PubMed]
- Fok JS, Katelaris CH, Brown AF, Smith WB. Icatibant in angiotensin-converting enzyme (ACE) inhibitor-associated angioedema. Intern Med J. 2015 Aug;45(8):821-7. [CrossRef] [PubMed]
- Riha HM, Summers BB, Rivera JV, Van Berkel MA. Novel therapies for angiotensin-converting enzyme inhibitor-induced angioedema: a systematic review of current evidence. J Emerg Med. 2017 Sep 19. pii: S0736-4679(17)30489-4. [CrossRef] [PubMed]
Cite as: Boudi FB, Rush JL, Farsar C, Chan CS. ACE inhibitor related angioedema: a case report and brief review. Southwest J Pulm Crit Care. 2017;15(4):165-8. doi: https://doi.org/10.13175/swjpcc114-17 PDF
Tumor Lysis Syndrome from a Solitary Nonseminomatous Germ Cell Tumor
Brandon T. Nokes, MD1
Rodrigo Cartin-Ceba, MD2
Joseph Farmer, MD2
Alyssa B. Chapital, MD, PhD2
1Hospital Internal Medicine and 2Division of Critical Care
Mayo Clinic Arizona
Phoenix, AZ USA
Abstract
Spontaneous tumor lysis syndrome is a rare clinical entity, which typically occurs in the context of rapidly proliferating hematologic malignancies. Tumor lysis syndrome in solid organ malignancies is even rarer, and typically provoked by cytotoxic treatment regimens. We describe a case of spontaneous tumor lysis of a solitary metastatic brain lesion from a nonseminomatous germ cell tumor. This case is unique in that spontaneous tumor lysis from a brain metastasis of a solid organ malignancy has never been reported, and spontaneous tumor lysis in a nonseminomatous germ cell tumor is exceedingly rare.
Case Report
A 31-year-old gentleman was admitted to our facility after developing status epilepticus and consequently, being involved in a MVA. Imaging revealed a 3.5cm right frontal brain lesion with surrounding edema, but no other acute intracranial pathology. The patient was intubated, sedated, and transferred to critical care for further treatment. His past medical history was notable for primary surgical resection of a T1N0M0 nonseminomatous germ cell tumor in March 2015, followed by detection of a 2.5cm lung nodule in September 2015, with concurrent beta-human chorionic gonadotropin (HCG) and alpha-fetoprotein (AFP) biochemical recurrence. He underwent 4 cycles of bleomycin, etoposide, and cisplatin (BEP).
A head CT revealed a 4cm x 3.5cm right frontal lesion with surrounding edema (Figure 1).

Figure 1. T2 Axial MRI showing 4 cm x 3.5 cm lesion with associated vasogenic edema.
Dexamethasone 4mg every 6 hours was initiated for treatment of vasogenic edema. Laboratory studies were significant for a white blood cell count elevated at 19.3 x109/L, international normalized ratio (INR) 1.34, partial thromboplastin time (PTT) 26.2 seconds, and prothrombin time (PT) 16.1 seconds. Plasma lactate was elevated at 30.6mmol/L. Bicarbonate was 6mmol/L with an anion gap of 45, glucose 186mg/dL, BUN 15.2mg/dL, and creatinine was 2.0mg/dL. Urine drug screen was negative. His AFP was 7.4ng/mL and beta-HCG was 13IU/L. Over the following 24 hours, the patient experienced decreased urine output. A bedside ultrasound reveals normal IVC collapse. Further lab assessment revealed a CK within normal limits and a urinalysis showed the presence of 11 to 20 RBCs, 4 to 10 WBCs and some granular casts as well as trace protein. His phosphorus was 8.9, calcium 8.1, and uric acid was 13mg/dL. His lactate dehydrogenase levels were also elevated at 271 U/L.
Due to concern of tumor lysis syndrome, the patient was initiated on rasburicase, which was followed by maintenance allopurinol 300mg daily. However, due to worsening renal failure, the patient was started on hemodialysis. He was taken to the operating room the following morning for immediate surgical resection of his brain metastasis; no evidence of residual disease was seen on follow-up imaging (Figure 2).

Figure 2. T2 Axial MRI status post a right frontal craniotomy and gross total resection of the previously noted mass. Small amount of blood noted within the resection cavity. Residual vasogenic edema persists in the white matter surrounding the operative bed.
Repeat chest, abdomen and pelvis imaging did not show any additional metastatic lesions.
In the following days, he was subsequently extubated, transferred to the floor, and continued hemodialysis, eventually fully recovering his renal function. Ultimately, he was discharged with outpatient follow-up for additional chemotherapy planning after physical rehabilitation.
Discussion
Tumor lysis syndrome (TLS) can be subdivided into laboratory TLS and clinical TLS, as defined by the Cairo-Bishop diagnostic criteria (1). Spontaneous TLS can occur in solid organ malignancies (1). TLS in solid organ malignancies is provoked by chemotherapy or radiation therapy, which creates massive cell lysis and elaboration of intracellular potassium, phosphate, and uric acid as well as hypocalcemia, which can lead to renal failure and cardiac dysrhythmias (1). LDH is also elevated. TLS can also be thought of as being provoked, either by ongoing chemotherapy or a decrease in effective circulating volume, or unprovoked. It is rare for TLS to occur in nonseminomatous germ cell tumors. Only 2 case reports have been published regarding spontaneous TLS in nonseminomatous germ cell tumors (2,3). Our case is most likely a spontaneous TLS. To date, no reports have been published regarding spontaneous TLS from a solitary brain metastasis from a nonseminomatous germ cell tumor. Further, no cases have been reported regarding tumor lysis from a solitary brain metastasis of any solid organ malignancy.
The occurrence of TLS in solid organ malignancies is thought to occur secondary to rapid cellular proliferation that exceeds the available blood supply for a tumor, leading to tumor ischemia and diffuse tumor cell necrosis. The biochemical milieu elaborated from these necrotic cells can result in end-organ pathology.
The treatment of TLS is contingent upon the rate of cancer progression and whether there is evidence of end-organ damage. Importantly and ideally, patients can be stratified into intermediate, moderate, or high-risk of developing TLS based on their malignancy type and rate of cancer progression, such that TLS may be prevented with prophylactic hydration, electrolyte monitoring and allopurinol or rasburicase (4,5). Biochemical TLS alone can be treated with IV hydration and allopurinol, a xanthine oxidase inhibitor which potentially halts TLS progression. When there is end-organ damage, rasburicase (a recombinant urate oxidase) is the first-line treatment along with aggressive hydration (5). Additional therapies are directed towards minimizing sequelae of TLS (i.e. calcium gluconate for hyperkalemia associated EKG changes or emergent dialysis for acute renal failure). There is no role for urinary alkalinization.
We were fortunate in that our patient had a great outcome, owing to early detection and aggressive intervention, and we implore our fellow physicians to be mindful of TLS as a possible clinical outcome in all malignancies, irrespective of its clinical rarity.
References
- Mirrakhimov AE, Ali AM, Khan M, Barbaryan A. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6(2):5389. [CrossRef] [PubMed]
- D'Alessandro V, Greco A, Clemente C, et al. Severe spontaneous acute tumor lysis syndrome and hypoglycemia in patient with germ cell tumor. Tumori. 2010;96(6):1040-3. [PubMed]
- Pentheroudakis G, O'Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9(7):554-7. [CrossRef] [PubMed]
- Feres GA, Salluh JI, Ferreira CG, Soares M. Severe acute tumor lysis syndrome in patients with germ-cell tumors. Indian J Urol. 2008;24(4):555-7. [CrossRef] [PubMed]
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16): 2767-78. [CrossRef] [PubMed]
Cite as: Nokes BT, Cartin-Ceba R, Farmer J, Chapital AB. Tumor lysis syndrome from a solitary nonseminomatous germ cell tumor. Southwest J Pulm Crit Care. 2017;15(4):148-50. doi: https://doi.org/10.13175/swjpcc107-17 PDF
October 2017 Critical Care Case of the Month
Margaret Ragland, MD1
Carolyn H. Welsh, MD1,2
Pulmonary Sciences and Critical Care Medicine
1University of Colorado Anschutz Medical Campus and 2VA Eastern Colorado Health Care System
Denver, Colorado USA
History of Present Illness
A 42-year-old man with a history of intravenous heroin abuse and chronic hepatitis C infection presents to the emergency department (ED) with recurrent abdominal pain. The pain was dull, epigastric, and did not radiate. The pain worsened after eating, but the timing after eating that it worsened was inconsistent. He had nausea but no vomiting. His bowel movements were normal without constipation, diarrhea, or melena.
He had presented to another ED multiple times with this same pain over the past six weeks. He does not know what the work-ups revealed, but was discharged from the emergency department each time. He received supportive care including fluids and analgesics, but the pain would always recur a few hours after returning home.
He went to a third ED a few weeks ago with bilateral testicular pain after which he was discharged home with acetaminophen for pain.
Past Medical History, Family History, and Social History
His past medical history is notable for bipolar disorder. He takes no prescribed medications and does not know his family’s medical history. He is a current every day smoker, has no history of heavy alcohol use, and uses intravenous heroin but no other recreational drugs.
Current Medications
Acetaminophen a few times a day for abdominal pain.
Review of Systems
He notes subjective fevers, poor appetite, and an 8 pound unintentional weight loss over the past six weeks.
Physical Exam
Vital signs are notable for hypertension to 158/91 mm Hg. Other vitals are within normal limits.
On exam, he is an ill appearing middle aged man who appears very uncomfortable. His abdomen is nondistended. He has normal bowel sounds and epigastric tenderness with a tender, smooth liver edge palpable just under the costal margin. He has decreased sensation to light touch in his toes with no skin changes. Toes are warm with capillary refill less than two seconds.
Laboratory Evaluation
CBC reveals a leukocytosis to 23,600 cells/mcL with 80% neutrophils; eosinophils are normal. Hemoglobin and platelet counts are normal. Sodium is 128 mmol/L with a bicarbonate of 30 mmol/L and creatinine of 0.64 mmol/L. AST 155 U/L, ALT 137 U/L, with a total bilirubin 1.1 mmol/L. Albumin is 1.8 g/L. INR is 1.9. Urinalysis showed 1+ protein.
What additional laboratory evaluation is indicated at this time? (Click on the correct answer to proceed to the second of six pages)
Cite as: Ragland M, Welsh CH. October 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;15(4):131-7. doi: https://doi.org/10.13175/swjpcc113-17 PDF
September 2017 Critical Care Case of the Month
James T. Dean III, MD
Tyler R. Shackelford, DO
Michel Boivin, MD
Division of Pulmonary, Critical Care and Sleep Medicine
University of New Mexico School of Medicine
Albuquerque, NM USA
Critical Care Case of the Month CME Information
Members of the Arizona, New Mexico, Colorado and California Thoracic Societies and the Mayo Clinic are able to receive 0.25 AMA PRA Category 1 Credits™ for each case they complete. Completion of an evaluation form is required to receive credit and a link is provided on the last panel of the activity.
0.25 AMA PRA Category 1 Credit(s)™
Estimated time to complete this activity: 0.25 hours
Lead Author(s): James T. Dean III, MD. All Faculty, CME Planning Committee Members, and the CME Office Reviewers have disclosed that they do not have any relevant financial relationships with commercial interests that would constitute a conflict of interest concerning this CME activity.
Learning Objectives: As a result of completing this activity, participants will be better able to:
- Interpret and identify clinical practices supported by the highest quality available evidence.
- Establish the optimal evaluation leading to a correct diagnosis for patients with pulmonary, critical care and sleep disorders.
- Translate the most current clinical information into the delivery of high quality care for patients.
- Integrate new treatment options for patients with pulmonary, critical care and sleep related disorders.
Learning Format: Case-based, interactive online course, including mandatory assessment questions (number of questions varies by case). Please also read the Technical Requirements.
CME Sponsor: University of Arizona College of Medicine
Current Approval Period: January 1, 2017-December 31, 2018
Financial Support Received: None
A 73-year-old man presented with a three-day history of diffuse abdominal pain, decreased urine output, nausea and vomiting. His past medical history included diabetes, coronary artery disease, hypertension and chronic back pain. The patient reported being started on hydrochlorothiazide, furosemide, pregabalin and diclofenac within the last week in addition to his long-standing metformin prescription.
Initial vitals were significant for tachypnea, tachycardia to 120 bpm, hypothermia to 35ºC and hypotension with a blood pressure of 70/40 mm Hg. Physical exam was remarkable for bilateral lung wheezing and significant respiratory distress. Laboratory examination was concerning for a pH of 6.85, pCO2 of < 5mmHg, serum lactate of 27mmol/l, WBC of 15.6 x106 cells/cc and a serum creatinine of 8.36 mg/dl. A chest X-ray showed evidence of mild pulmonary edema and a CT of the abdomen did not show any acute pathology.
What is the most likely etiology of the patient’s severe acidosis? (Click on the correct answer to proceed to the second of four pages)
Cite as: Dean JT III, Shackelford TR, Boivin M. September 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;15(3):100-3. doi: https://doi.org/10.13175/swjpcc101-17 PDF
August 2017 Critical Care Case of the Month
Kolene E. Bailey, MD1
Carolyn Welsh, MD1,2
Pulmonary Sciences and Critical Care Medicine
1University of Colorado Anschutz Medical Campus and 2VA Eastern Colorado Health Care System
Denver, CO USA
History of Present Illness
The patient is a 26-year-old woman with who was admitted to the hospital for second cycle of chemotherapy for a large mediastinal synovial sarcoma diagnosed 2 months prior to admission. Symptoms started 6 months prior to presentation with cough. She related the cough to her cigarette smoking and quit. Upon persistence of symptoms, she was evaluated by her physician who ordered imaging. Work-up revealed a large 12 x 14cm synovial sarcoma with internal necrosis that encased the subclavian artery, and descending thoracic aorta, inseparable from pericardium and left atrium. It also encased the pulmonary veins, pulmonary arteries, and airways. Malignancy was complicated by extensive left upper extremity DVT for which she has been on anticoagulation since her last admission, SVC syndrome, and severe mucositis.
Past Medical History, Family History, and Social History
She has a past medical history significant for malignant melanoma surgically resected 7 years previously, as well as generalized an anxiety disorder.
Her family history includes a maternal grandfather with esophageal cancer and maternal great-grandmother with pancreatic cancer. She is single and lives with her parents. She is a former 8 pack year smoker, and daily edible marijuana user. She worked as a hairdresser, but is now unable to work.
Current Medications:
- Escitalopram (Lexapro) 10mg PO daily
- Dalteparin
- Oxycontin 10mg PO BID + Oxycodone 5-10mg PO Q4H PRN pain
- Antiemetics: Compazine PRN, Ondansetron PRN, dexamethasone 4mg BID for 3 days following chemotherapy
- Lorazepam 1mg PO Q4H PRN anxiety
- Pegfilgastrim after chemotherapy
- Senna 3 tabs in AM, 2 tabs in PM
Hospital Course
After starting cycle #2 of chemotherapy (doxorubicin, ifosfamide, and mesna), she experienced significant nausea and anxiety and was prescribed scheduled ondansetron/dexamethasone, prochlorperazine, promethazine and lorazepam. The night of hospital day #2, her providers noticed altered mental status and unusual behavior. They asked her draw a clock which is shown (Figure 1).

Figure 1. Clock drawn by patient.
What is on your differential diagnosis for this patient’s altered mental status? (Click on the correct answer to proceed to the second of five pages)
- Delirium
- Ifosfamide-induced encephalopathy
- Toxic-metabolic encephalopathy secondary to the medications received
- 1 and 3
- All of the above
Cite as: Bailey KE, Welsh C. August 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;15(2):61-6. doi: https://doi.org/10.13175/swjpcc094-17 PDF
Telemedicine Using Stationary Hard-Wire Audiovisual Equipment or Robotic Systems in Critical Care: A Brief Review
Nidhi S. Nikhanj, MD1,2
Robert A. Raschke, MD1,2
Robert Groves, MD1,2
Rodrigo Cavallazzi, MD3
Ken S. Ramos, MD1
1Arizona College of Medicine-Phoenix
Phoenix, AZ USA
2Banner University Medical Center-Phoenix
Phoenix, AZ USA
3University of Louisville School of Medicine
Louisville, KY USA
A shortage of critical care physicians in the United States has been widely recognized and reported (1). Most intensive care units (ICUs) do no not have a formally-trained intensivist in their staff despite compelling evidence that high-intensity intensivist staffing leads to better patient outcomes (1,2). Critical care telemedicine is one potential solution that has expanded rapidly since its inception in 2000 (3). In its simplest form, telemedicine leverages audiovisual technology and the electronic medical record to provide remote two-way communication between a physician and a patient. Current telemedicine models differ by the type of hardware facilitating remote audiovisual interaction, the location of the provider, and the type of patient-care service provided. We collectively have experience with several of these models and feel that future telemedicine programs will likely integrate the most advantageous aspects of each with an increasing role for telemedicine robotics.
The dominant current model for providing critical care telemedicine in large healthcare systems utilizes stationary hard-wired audiovisual equipment linking each ICU room to a centralized control location (4). Typically, this control center provides surveillance of a large number of patients using computerized decision support software linked to the EMR – a single physician can cover approximately 100 patients with the appropriate support infrastructure. This model also provides the ability to remotely “round” on ICU patients and to quickly respond to questions posed by nursing or medical emergencies across a broad geographic range. This approach requires a high up-front capital cost approximated at 50-100K per hospital bed covered (5).
Data supporting the benefit of this model of ICU telemedicine has been mixed, but several considerations are important in appraising the literature. A double-blinded RCT for ICU telemedicine intervention is not feasible. Heterogeneity in clinical workflows and staffing models across the country should be considered when assessing the internal validity and generalizability of published studies. For instance, Thomas and colleagues concluded that a telemedicine ICU service resulted in no overall improvement in mortality or length of stay (LOS) (6), but the tele-intensivists in the study were limited by only being allowed to intervene in the care of less than a third of the study patients. Nassar and colleagues published a negative study in a healthcare system in which resident and attending physicians were already available in-house for overnight patient care (7). Likely, the potential benefit of a telemedicine program can be optimized in a clinical setting in which other physicians are not physically available at the locality 24/7 and telemedicine intensivists are allowed to appropriately intervene when indicated.
Despite these difficulties, there is a growing body of evidence that suggests a centralized telemedicine ICU model is effective in a number of areas including: improvements in compliance with evidence based practices (8, 9), increased job satisfaction of ICU nurses (10) and reduction in the cost of care of the sickest patients in the institutional setting (11). Other studies suggest that a telemedicine platform can reduce mortality and LOS by allowing for earlier intensivist involvement, promoting adherence to best practices, shortening alarm response times and improving access to ICU performance data that can be used to drive continuous quality improvement (12,13).
Commercially available telemedicine robots are mobile units equipped with a digital camera, microphone and monitor screen that provides two-way audiovisual communications with the control center via a wireless internet connection (14). Telemedicine robots can be operated with much lower initial capital costs - for instance, an ICU group at a large acute care hospital might provide coverage at a rural healthcare setting using a single robot (15). Such a system can be used for daily rounding or for reactive consultation. Like hard-wired systems, telemedicine robots have been shown to be well accepted by providers (16) and patients (17), and their use has been associated with reduced ICU length-of-stay and decreased delay in response to clinical events by the physician (18).
Telemedicine robotic systems have several disadvantages – they do not provide large-scale EMR surveillance leveraging computerized decision support logic and they are significantly less efficient than hard-wired systems for high-volume patient care since they have to physically relocate from patient room to patient room. However, unique capabilities of telemedicine robots are being developed that cannot be duplicated by hard-wired systems. Telemedicine robots can be equipped with a digital stethoscope (19). They can perform physical examination elements that require tactile communication – such as the determination of the Glasgow coma scale (20). A robotic arm can be used to remotely perform point-of-care ultrasonography. This has been successfully operationalized for cardiac, abdomino-pelvic, and vascular indications (21,22). Telemedicine robots have been developed that can place peripheral or central venous catheters (23). The development of surgical robots that incorporate tomographic capability and that can perform battlefield stabilization procedures in either autonomous or teleoperative modes (24) provide a glimpse of the potential for telemedicine robots in the ICU.
Although healthcare systems currently implementing telemedicine services will likely choose either a hard-wired or a robotic model – largely based on cost and the volume of required services - we believe the optimal telemedicine system of the future will and should incorporate both technologies. Real-time data acquisition coupled with ready access to timely interventions constitute the basis for faster deployment of precision health care strategies in the ICU setting.
References
- Kelley MA, Angus D, Chalfin DB, Crandall ED, et al. The critical care crisis in the United States: A report from the profession. Chest. 2004;125:1514-7. [CrossRef] [PubMed]
- Pronovost PJ, Angus DC, Dorman T, Robinson KA, et al. Physician staffing patterns and clinical outcomes in critically ill patients. JAMA. 2002;288:2151-62. [CrossRef] [PubMed]
- Rosenfeld BA, Dorman T, Breslow MJ, et al. Intensive care unit telemedicine: alternate paradigm for providing continuous intensivist care. Crit Care Med. 2000;28:3925-31. [CrossRef] [PubMed]
- Kahn JM, Cicero BD, Wallace DJ, Iwashyna TJ. Adoption of intensive care unit telemedicine in the United States. Crit Care Med. 2014;42:362-8. [CrossRef] [PubMed]
- Kumar G, Falk DM, Bonello RS, et al. The costs of critical care telemedicine programs: A systematic review and analysis. Chest. 2013;143:19-29. [CrossRef] [PubMed]
- Thomas EJ, Lucke JF, Wueste L. Association of telemedicine for remote monitoring of intensive care patients weith mortality, complications and length of stay. JAMA. 2009;302:2671-78. [CrossRef] [PubMed]
- Nassar BS, Vaughan MS, Jiang L, Reisinger HS, et al. Impact of an intensive care unit telemedicine program on patient outcomes in an integrated health care system. JAMA Intern Med. 2014;174:1160-7. [CrossRef] [PubMed]
- Ventataraman R, Ramakrishnan N. Outcomes related to telemedicine in the intensive care Unit. Crit Care Clinics 2015;31:225-37. [CrossRef] [PubMed]
- Youn BA. ICU process improvement using telemedicine to enhance compliance and documentation for the ventilator bundle. Chest. 2006;130:(meeting abstracts) 226S-c.
- Hoonakker PL, Carayon P, McGuire K, et al. Motivation and job satisfaction of tele-ICU nurses. J Crit Care. 2013;28:890-901. [CrossRef] [PubMed]
- Franzini L, Sail KR, Thomas EJ, et al. Costs and cost-effectiveness of a telemedicine intensive care unit program in six intensive care units in a large health care system. J Crit Care. 2011;26:329e1-6. [CrossRef] [PubMed]
- Lilly CM, Cody S, Zhao H. Hospital mortality, length of stay and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305:2175-83. [CrossRef] [PubMed]
- Lilly CM, Zubrow MT, Kempner KM, Reynolds H, et al. Critical Care telemedicine: Evolution and state of the art. Crit Care Med. 2014;42:2429-36. [CrossRef] [PubMed]
- Chung KK, Grathwohl KW, Poropatich RK, Wolf SE, et al. Robotic telepresence: Past present and future. Journal of Cardiothoracic and Vascular Anesthesia. 2007;21:593-6. [CrossRef] [PubMed]
- Murray C, Ortiz E, Kubin C. Application of a robot for critical care rounding in small rural hospitals. Crit Care Nurs Clin North Am. 2014;26:477-85. [CrossRef] [PubMed]
- Reynolds EM, Grujovski A, Wright T, Foster M, Reynolds HN. Utilization of robotic remote presence technology within North American intensive care units. Telemedicine and e-health. 2012;18:507-15. [CrossRef] [PubMed]
- Sucher JF, Todd SR, Jones SL, Throckmorton T, et al. Robotic telepresence: A helpful adjunct that is viewed favorably by critically ill surgical patients. Am J Surg. 2011;202:843-7. [CrossRef] [PubMed]
- Vespa PM, Miller C, Hu X, Nenov V, et al. Intensive care unit robotic telepresence facilitates rapid physician response to unstable patients and decreased cost in neurointensive care. Surgical Neurology. 2007;67:331-7. [CrossRef] [PubMed]
- Lakhe A, Sodhi I, Warrier J, Sinha V. Development of digital stethoscope for telemedicine. J Med Eng Technol. 2016;40:20-4. [CrossRef] [PubMed]
- Adcock AK, Kosiorek H, Parich P, Chauncey A, Wu Q, Demaerschalk BM. Reliability of robotic telemedicine for assessing critically ill patients with the full outline of unresponsiveness score and Glasgow coma scale. Telemed J E Health. 2017 Jan 13. [CrossRef] [PubMed]
- Avgousti S, Panayides AS, Jossif AP, Christoforou EG, et al. Cardiac ultrasonography over 4G wireless networks using a tele-operated robot. Healthc Technol Lett. 2016;3:212-7. [CrossRef] [PubMed]
- Georgescu M, Sacccomandi A, Baudron B, Arbeille PL. Remote sonography in routine clinical practice between two isolated medical centers and the university hospital using a robotic arm: A 1-year study. Telemed J E Health. 2016;22:276-81. [CrossRef] [PubMed]
- Kobayashi Y, Hong J, Hamano R, Okada K, Fujie MG, Hashizume M. Development of a needle insertion manipulator for central venous catheterization. Int J Med Robot. 2012;8(1):34–44. [CrossRef] [PubMed]
- Garcia P, Rosen J, Kapoor C, Noakes M, et al. Trauma Pod: a semi-automated telerobotic surgical system. Int J Med Robot. 2009;5:136-46. [CrossRef] [PubMed]
Cite as: Nikhanj NS, Raschke RA, Groves R, Cavallazzi R, Ramos KS. Telemedicine using stationary hard-wire audiovisual equipment or robotic systems in critical care: a brief review. Southwest J Pulm Crit Care. 2017;15(1):50-3. doi: https://doi.org/10.13175/swjpcc087-17 PDF
Carotid Cavernous Fistula: A Case Study and Review
Iaswarya Ganapathiraju, OMS-IV1
Douglas T Summerfield, MD2
Melissa M Summerfield, MD2
1Des Moines University College of Osteopathic Medicine
Des Moines, IA USA
2Mercy Medical Center North Iowa and North Iowa Eye Clinic
Mason City, IA USA
Abstract
Carotid cavernous fistulas are rare complications of craniofacial trauma, resulting in abnormal connections between the arterial and venous systems of the cranium. The diagnosis of carotid cavernous fistulas and other injuries as a result of trauma can be confounded by the traumatized patient’s inability to communicate their symptoms to their physician. The following case study demonstrates the importance of a thorough physical exam in caring for such patients and serves to remind physicians to have a low threshold for consultation when managing numerous injuries following trauma.
Introduction
Carotid cavernous fistulas (CCFs) are aberrant connections between the carotid arterial system and the cavernous sinus, which form as complications of craniofacial trauma, or are congenital or spontaneous in nature (1). They occur in up to 3.8% of patients with basilar skull fractures and are more common with middle fossa fracture (2). Prompt diagnosis and treatment of CCF is necessary as approximately 20 – 30% of carotid cavernous fistulas lead to vision loss if not addressed appropriately (3)/\The following is a case study of a patient who presented with multiple traumatic injuries including CCF with subsequent discussion of the typical presentation, diagnosis, and treatment of direct CCF.
Case Presentation
A 64-year-old woman with a therapeutic INR on Coumadin for atrial fibrillation sustained a fall down a flight of stairs. She was found unresponsive the next day by her relatives and was subsequently brought to the emergency department for evaluation. A maxillofacial CT showed a nondisplaced right maxillary wall fracture and nondisplaced zygomatic arch fracture, as well as a subtle inferotemporal orbital fracture, none of which was determined to require immediate treatment by the otolaryngology service. Further imaging included a CT of the head which revealed a large subdural hematoma, a superotemporal hematoma, and subfalcine herniation. She was taken to the OR for emergent craniotomy and evacuation of the hematoma before transfer to the critical care unit. In the CCU, she remained intubated and sedated but her condition improved until extubation on hospital day 3. She continued to have swelling surrounding both eyes during this time, but physical exam showed pupils which were equal, round, and reactive to light.
On day 6 of her stay, the patient was noted to have waxing and waning confusion and slightly increased oxygen requirement. Thus, she was re-intubated and sedated for “agitation” and “hypoxic respiratory failure.” Physical exam on the next day was notable for pupillary anisocoria with the right pupil at 1 mm diameter and left at 2.5 mm. There was a poor pupillary light reaction bilaterally. Neurology was consulted and recommended repeat imaging and EEG. Repeat CT and MRI of the brain showed no evidence of herniation, and EEG was negative for seizure-like activity. The anisocoria was thought to be from mass effect of the temporal lobe on cranial nerve III. The patient’s condition continued to deteriorate; physical exam elicited grimace to painful stimuli and the patient was able to open her eyes but did not track movement or follow commands. She was subsequently noted to have a left orbit that became harder to compress with ballottement test compared to the right, so Ophthalmology was consulted.
An ophthalmologic exam showed extensive chemosis of the left eye compared to the right with conjunctival hemorrhage in bilateral eyes (Figure 1).

Figure 1. Ophthalmologic exam revealed chemosis, exophthalmos, and a mid-dilated, fixed pupil of left eye compared to right.
Ocular tonometry revealed a pressure of 14 mmHg in the right eye and 53 mmHg in the left. There was a mid-dilated, fixed pupil on the left. The differential at this point included traumatic acute angle closure glaucoma versus a retroorbital process. The patient was started on timolol, pilocarpine, and dorzolamide eye drops for intraocular pressure control. An orbital CT was obtained, which showed an engorged superior ophthalmic vein on the left with a new 4 mm proptosis of the left eye (Figure 2) when compared to previous imaging.

Figure 2. A: CT scan showed proptosis of 4 mm of left eye compared to right eye. B: Enlarged left ophthalmic vein also noted on CT scan (arrow).
This raised concern for traumatic carotid cavernous fistula. A CTA obtained the following morning confirmed this suspicion (Figure 3).


Figure 3. A: Reconstructed coronal CT coronal angiogram showing enlarged left cavernous sinus, confirming diagnosis of carotid cavernous fistula. B-E: Static coronal images from CT angiogram with major arteries labeled. F: Video of CT angiogram.
The patient was transferred to an outside facility for surgical management, which consisted of angiography and embolization via coiling of her CCF.
Discussion
Carotid cavernous fistulas are abnormal connections that form between the cavernous sinus and the internal or external carotid arteries, or branches of the internal or external carotid arteries. They are divided into direct and indirect variants per Barrow classification (Table 1, Figure 4).

ICA = Internal carotid artery ECA = External carotid artery

Figure 4. A: The normal eye: superior ophthalmic vein draining into cavernous sinus and internal and external carotid arteries traversing the cavernous sinus. B: Barrow Classifications for types of carotid cavernous fistulas: Type A: direct connection between internal carotid artery and cavernous sinus. Type B: connection between dural branches of internal carotid artery and cavernous sinus. Type C: connection between dural branches of external carotid artery and cavernous sinus. Type D: connection between dural branches of both internal carotid artery and external carotid artery and the cavernous sinus.
Types B through D are commonly termed ‘indirect’ or ‘dural’ fistulas. These can develop spontaneously as a result of hypertension and are the more common presentation of CCF. More specifically, type B is a connection between the dural branches of the ICA and the cavernous sinus, type C is a connection between the dural branches of the external carotid artery (ECA) and the cavernous sinus, and type D connects the dural supply of both the ICA and ECA and the cavernous sinus (1). Type A, or a ‘direct’ CCF, is a connection between the intracavernous internal carotid artery (ICA) and the cavernous sinus. Direct CCF is a rare ocular complication that forms most commonly as a result of craniofacial trauma, but can also be due to aneurysmal rupture or spontaneous development. This is also the most dramatic presentation of CCF and was the case in our patient.
Prompt identification and management of CCF is necessary to prevent associated morbidity and mortality. The presentation of CCF depends mainly on the drainage of the fistula. Anterior-drainage of fistulas through the superior ophthalmic vein produces symptoms of exophthalmos, proptosis, acute chemosis or swelling/edema of conjunctiva, and headache, all of which are more common in direct CCFs. The backup of drainage can result in a secondary angle closure with extremely high intraocular pressure. Posterior-drainage of fistulas into the superior and inferior petrosal sinuses tend to lack the aforementioned features of orbital congestion, but can produce painful cranial neuropathy of the trigeminal, facial, or ocular motor nerves. Failure to identify and appropriately treat posterior-draining fistulas can lead to eventual reversal of flow and development of anterior drainage (4).
The signs of CCF are not visible on neuroimaging at a patient’s presentation and generally develop over the first week a patient is admitted. Clinical signs which may prompt further investigation and repeat imaging include chemosis, increasing exophthalmos, pain, and increased intraocular pressure. Often, the tools for checking intraocular pressure are not available in an ICU setting. In the absence of signs of a ruptured globe, an intensivist could palpate the orbit over a closed eye (as occurred in this case). If there is asymmetry in resistance to palpation, this should incite an ophthalmologic consult to consider a retro-orbital process.
Repeat neuroimaging is likely to be done in these cases, but it is important to order the right test. Radiologic signs of CCF include proptosis and asymmetric enlargement of a cavernous sinus or superior ophthalmic vein and would be noted on an orbital or maxillofacial CT. A head CT might miss these signs, so it is important to obtain imaging dedicated to examining the retro-orbital space. To confirm the diagnosis of CCF, one must then obtain a CT angiogram, which will show the aberrant connections between the intracranial vessels. Upon confirming a diagnosis of CCF, the preferred mode of management is endovascular obliteration using an arterial or venous approach as it has been shown to be safe and effective, and confers long-term cure in most cases (5).
A previous review of 16 cases of carotid cavernous fistulas treated with transarterial embolization with detachable balloon show satisfactory results, defined as resolution of CCF without residual disability, in 11 cases and resolution but with residual disability in 5 cases. The most common of the disabilities in these cases was vision impairment, as seen in 4 out of the 5 cases. In addition, 14 out of the 16 cases resolved with preserved internal carotid artery flow (1). As a result, transarterial embolization with detachable balloon (TAEDB) has been established as the preferred method of treatment for carotid cavernous fistulas (6). Other options for treatment include neurosurgery and stereotactic radiosurgery when endovascular approach is not feasible.
Our patient presented with several traumatic injuries following a fall down a flight of stairs and was unable to contribute to history-taking. Detection and treatment of the CCF that she later developed was complicated by several factors. The true exophthalmos of the affected eye was partially masked by the fact that she had an inferotemporal orbital fracture of the opposite eye, which was incorrectly thought to be enophthalmic. Additionally, her altered mental status and subsequent re-intubation limited her ability to vocalize the pain which would have been present in her affected eye due to tremendously increased intraocular pressure.
From a critical care physician perspective, part of the key to her diagnosis was her re-intubation. The patient developed severe agitation requiring sedation without other more typical reasons for intubation such as hypoxia, tachypnea, or dyssynchronous breathing. We suspect this agitation was likely secondary to pain from the rapidly increasing pressure in her affected eye which became symptomatic just prior to her worsening mental status. Her physical exam was ultimately crucial to the detection of her CCF, specifically chemosis, exophthalmos, and increased intraocular pressure in the affected eye. These signs led to the subsequent ophthalmologic consultation, imaging, and eventually the diagnosis of CCF.
An important lesson learned from this patient’s management is having a low threshold for consultation when the clinical picture does not match diagnostic workup. In our case, the patient’s clinical condition changed but repeat workup including EEG and MRI of the head was negative. Previous imaging had revealed right-sided facial fractures, yet her new findings, including increased resistance to palpation of the orbit and chemosis, were largely left-sided. In situations when the cause of a patient’s deteriorating condition is unclear and there is incongruity between the physical exam and diagnostic workup, it is imperative to obtain further consultation. In our case, the ophthalmic exam gave the clues for further workup and the ultimate diagnosis.
In conclusion, this patient’s case is a good study in the classic presentation of direct CCF in association with craniofacial trauma, and also illuminates the difficulty in detection of orbital injuries in a trauma patient who cannot vocalize the symptoms they are experiencing. The lesson learned from her presentation is to have a low threshold for ophthalmologic consultation for unexplained changes in ophthalmic condition and discrepancies between clinical presentation and diagnostic findings.
References
- Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985 Feb;62(2):248-56. [CrossRef] [PubMed]
- Liang W, Xiaofeng Y, Weiguo L, Wusi Q, Gang S, Xuesheng Z. Traumatic carotid cavernous fistula accompanying basilar skull fracture: a study on the incidence of traumatic carotid cavernous fistula in the patients with basilar skull fracture and the prognostic analysis about traumatic carotid cavernous fistula. J Trauma. 2007 Nov;63(5):1014-20. [CrossRef] [PubMed]
- Doran M. Carotid-Cavernous Fistulas: Prompt Diagnosis Improves Treatment. American Academy of Ophthalmology. https://www.aao.org/eyenet/article/carotid-cavernous-fistulas-prompt-diagnosis-improv. Published March 18, 2016. Accessed July 11, 2017.
- Miller NR. Diagnosis and management of dural carotid-cavernous sinus fistula. Neurosurg Focus. 2007;23(5):E13. [PubMed]
- Gupta AK, Purkayastha S, Krishnamoorthy T, Bodhey NK, Kapilamoorthy TR, Kesavadas C, Thomas B. Endovascular treatment of direct carotid cavernous fistulae: a pictorial review. Neuroradiology. 2006 Nov;48(11):831-9. [CrossRef] [PubMed]
- Lewis AI, Tomsick TA, Tew JM Jr, Lawless MA. Long-term results in direct carotid-cavernous fistulas after treatment with detachable balloons. J Neurosurg. 1996 Mar;84(3):400-4. [CrossRef] [PubMed]
Cite as: Ganapathiraju I, Summerfield DT, Summerfield MM. Carotid cavernous fistula: a case study and review. Southwest J Pulm Crit Care. 2017:15(1):32-8. doi: https://doi.org/10.13175/swjpcc083-17 PDF
July 2017 Critical Care Case of the Month
Robert A. Raschke, MD
Banner University Medical Center Phoenix
Phoenix, AZ USA
History of Present Illness
A 62-year-old man was brought to the Emergency Department with an altered mental status after a neighbor found him unresponsive. Medications the paramedics found in his home were cyclobenzaprine, duloxetine, gabapentin, levothyroxine, ibuprofen, and tramadol.
Past Medical History, Social History and Family History
He had a past medical history of neck and back pain and hypothyroidism. He lived alone. There was a history of a C3-4 anterior cervical discectomy in 2010. Other history including family history was unobtainable.
Physical Examination
- Vital Signs: HR 61 beats/min, BP 86/50 mm Hg, RR 8 breaths/min, T 32.2º C
- General: arousable but did not answer questions. He had multiple tattoos. No needle track marks are identified.
- HEENT: pupils were small but reacted to light.
- Lungs: clear to auscultation.
- Heart: regular rhythm without murmur.
- Abdomen: soft without organomegaly or masses.
- Neurology: he moved all 4 extremities but minimally. Plantar reflexes were downgoing.
Which of the following should be done immediately? (Click on the correct answer to proceed to the second of six pages)
Cite as: Raschke RA. July 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;15(1):7-14. doi: https://doi.org/10.13175/swjpcc081-17 PDF
High-Sensitivity Troponin I and the Risk of Flow Limiting Coronary Artery Disease in Non-ST Elevation Acute Coronary Syndrome (NSTE-ACS)
Ali Abdul Jabbar, MD 1,3,4
Omar Mufti, MD1
Sayf Altabaqchali, MD RPVI4
Chowdhury Ahsan, MD PhD2
Mohanad Hasan, MD2
Ronald Markert, PhD1
Bryan White, MD1
George Broderick, MD1
1Cardiology Division, Department of Internal Medicine, Wright State University Boonshoft School of Medicine, Dayton, Ohio.
2Cardiology Division, Department of Internal Medicine, University of Nevada School of Medicine, Las Vegas, Nevada.
3Department of Cardiovascular Medicine, University of Toledo Health Science Campus, Toledo, Ohio.
4Department of Cardiology, Ochnser Heart and Vascular Institute, New Orleans, Louisiana.
Abstract
Background: In acute coronary syndrome, elevated troponins are associated with worse clinical outcomes. We examined the relationship between the level of troponin elevation and the presence of a flow-limiting lesion for patients with no history of coronary disease admitted with NSTE-ACS.
Methods: From January of 2010 until April of 2013, 561 patients received coronary angiography for new-onset NSTE-ACS. The Mann-Whitney Test, chi-square test, and Spearman correlation were used to examine relationships. Inferences were made at the 0.05 level of significance. The independent samples t test and the chi square test were used to identify predictors of LV systolic dysfunction- LVSD.
Results: The 430 patients with a flow-limiting coronary lesions had a higher troponin I level than the 131 patients without obstructive coronary disease (5.69 ng/ml vs. 2.85 ng/ml, p=0.002). Further, within troponin categories, those in the greater than 5.0 ng/ml group were more likely to have angiographically significant CAD than those in the less than 0.5 ng/ml group (p=0.012). Elevated troponins were also associated with increased thrombus burden, worse systolic function, higher complexity of the lesions, and worse post intervention TIMI flow. Cardiac troponin >5ng/ml [odds ratio=2.13 (95%CI=1.22 to 3.70) p=0.008] and DM [odds ratio=1.74 (95%CI=1.02 to 2.97) p=0.042] were independent predictors of LVSD. Advanced LM disease and age were marginally significant.
Conclusion: The degree of cardiac troponin I elevation should be incorporated into the risk stratification models of NSTE-ACS to promptly triage high-risk patients to early invasive strategies and tailored anticoagulant therapy to reduce troponin elevation and improve myocardial perfusion.
Background
Cardiac troponin is the main biomarker of myocardial ischemia. In acute coronary syndrome, elevated troponin levels are associated with complex obstructive coronary anatomy and impaired myocardial tissue perfusion. Elevated troponins can identify high-risk patients with non-ST elevation acute coronary syndrome (NSTE-ACS), who may benefit from early invasive management. However, the degree of troponin elevation has not been incorporated in risk stratification models for NSTE-ACS. To triage patients for conservative versus invasive management strategies, we need to define the significance of the magnitude of troponin elevation following NSTE-ACS (1).
NSTE-ACS is the most common form of acute coronary syndrome. Troponin elevation signifies a delayed presentation in ST elevation MI not so for NSTE-ACS. The determination of ischemic injury timing becomes more challenging when NSTE-ACS patient present with variable levels of troponin elevation.
Thus, we examined the relationship between troponin levels and the extent of coronary disease and myocardial dysfunction, as assessed by coronary angiography, in a subset of patients with no history of coronary disease admitted with NSTE-ACS.
Methods
Study design
This is a retrospective study of a cohort admitted to a university-affiliated teaching hospital with highly specialized cardiovascular care over a period of 40 months. The data for this study were obtained from the National Cardiovascular Data Registry (NCDR) database and electronic chart review of study participants.
Serum cardiac troponin levels were measured using a high-sensitivity enzyme-linked immune-absorbent assay kit (VITROS® Troponin I ES Assay, © Ortho Clinical Diagnostics, Johnson & Johnson -Hong Kong- Ltd. 2003-2014). A level greater than 0.033 ng/ml is considered above the reference range and represents a positive test value. The highest troponin I level prior to coronary angiography was used in the analyses.
Selection of study participants
The study investigated the association of cardiac troponin I levels and the presence of a flow-limiting coronary arterial lesion; a flow-limiting lesion was defined as an angiographically significant coronary lesion warranting percutaneous and/or surgical revascularization. The study only included patients with new-onset (de novo) NSTE-ACS. Patients with a history of coronary artery disease, heart failure, and cardiac bypass were excluded.
Study Objectives and Data Analysis
In addition to determining the association between cardiac troponin I levels and the presence of flow-limiting coronary artery disease, the study also examined the relationship between cardiac troponin I levels and various other factors, including vascular anatomy, lesion complexity, success of percutaneous intervention (based on the post-intervention Thrombolysis In Myocardial Infarction - TIMI - study grading system of the coronary blood flow), and incidence of Left Ventricular Systolic Dysfunction (LVSD), defined by an ejection fraction of less than 40% on left ventriculogram, in de-novo NSTE-ACS patients.
Means and standard deviations are reported for continuous variables, and counts and percents for categorical variables. The independent samples Mann-Whitney Test (two groups), Kruskal-Wallis Test (three groups), one-way analysis of variance (ANOVA) with Least Significance Difference post hoc test, chi square test, and Spearman correlation were used to examine relationships. Inferences were made at the 0.05 level of significance with no corrections for multiple comparisons. Multivariable logistic regression was used to determine if troponin is an independent risk factor for LVSD. Analyses were conducted using IBM SPSS Statistics 22.0 (IBM, Armonk, NY).
Results
Baseline characteristics
From January 2010 through April 2013, 561 patients received coronary angiography for new onset NSTE-ACS. Of this total, 485 (86.5%) had left ventricular functional assessment at the time of cardiac catheterization. All patients were managed invasively.
Patients were divided into three groups according to the degree of troponin I elevation (mild <0.5 ng/ml [n = 167], moderate 0.5-5 ng/ml [n = 263], and high >5 ng/ml [n = 131]). Table 1 shows that age differed among the three groups (p = 0.008): the moderate group was older than the mild group (mean age = 66.3±13.9 vs. 62.2±12.5) but not the high groups (64.1±13.3).
Table 1. Characteristics of troponin groups.

Abbreviations - GFR: glomerular filtration rate; PCI: percutaneous coronary artery intervention; IABP: intra-aortic balloon pump; UH: unfractionated heparin; LMWH: low molecular weight heparin
a The moderate group was older than the mild group (mean age = 66.3±13.9 vs. 62.2±12.5) but not the high group (64.1±13.3).
bPatients were more likely to be Caucasian as troponin categories increased (66.5% for the <0.5 ng/ml group, 78.2% for the 0.5-5 ng/ml group, 81.5% for the >5.0 ng/ml group) and less likely to be African American as troponin categories increased (32.3% for the <0.5 ng/ml group, 21.0% for the 0.5-5 ng/ml group, 17.7% for the >5.0 ng/ml group); p value for chi square test excludes Asians and Hispanics due to low counts.
cThe moderate and high groups had a higher euroscore than the mild group (mean euroscore = 5.44±3.3 and 5.98±6.1 vs. 4.38±2.8).
dPatients were more likely to receive unfractionated heparin as troponin categories increased (63.4% for the <0.5 ng/ml group, 69.7% for the 0.5-5 ng/ml group, 84.2% for the >5.0 ng/ml group).
Patients were more likely to be Caucasian as troponin categories increased (66.5% for the <0.5 ng/ml group, 78.2% for the 0.5-5 ng/ml group, 81.5% for the >5.0 ng/ml group) and less likely to be African American as troponin categories increased (32.3% for the <0.5 ng/ml group, 21.0% for the 0.5-5 ng/ml group, 17.7% for the >5.0 ng/ml group).
The moderate and high groups had a higher euro-score than the mild group (mean euro-score = 5.44±3.3 and 5.98±6.1 vs. 4.38±2.8 p = 0.003). Patients were more likely to have been treated with unfractionated heparin as troponin levels increased (63.4% for the <0.5 ng/ml group, 69.7% for the 0.5-5 ng/ml group, 84.2% for the >5.0 ng/ml group (p = 0.01).
Primary outcomes
Patients with flow-limiting coronary lesions (n = 430) had higher mean troponin I levels than patients without obstructive coronary disease (n = 131) [5.69±12.57 ng/ml vs. 2.85±5.76 ng/ml, p = 0.002]. More importantly, the proportion of patients with angiographically significant CAD increased as troponin levels increased (70.7% for the <0.5 ng/ml group, 77.2% for the 0.5-5 ng/ml group, 83.2% for the >5.0 ng/ml group (p = 0.038) (Figure 1).

Figure 1. Troponin groups and the presence of flow-limiting CAD.
Secondary outcomes
Elevated troponin levels were associated with increased thrombus burden (8.34±15.44 ng/ml for patients with intracoronary thrombus vs 5.29±13.11 ng/dl for those without thrombotic lesions, p = 0.001), worse systolic function (6.62+9.77 ng/dl for those with LVEF <40% compared to 4.42+8.70 ng/dl for those with preserved LV function, p=0.003), higher complexity of the lesions (patients with high - type C – lesions, per AHA/ACC classification, had mean troponin level of 8.38±17.71 ng/ml vs 3.44±7.7 ng/ml for those with non-high - type C - lesions, p < 0.001), and worse TIMI flow (patients with TIMI grade 0 flow post-intervention had mean troponin of 49.1±71.99 ng/ml vs 5.16±10.41 ng/ml for those with TIMI grade 3 flow, p = 0.017) post intervention.
Patients with LVSD were more likely to be older, have diabetes (DM), have more advanced left main coronary disease, and have cardiac troponin levels greater than 5 ng/ml. When the statistically significant predictors for LVSD (p<0.05) from the univariate analysis were entered into a multivariable logistic regression model of analysis, cardiac troponin levels > 5ng/ml [odds ratio = 2.13 (95%CI = 1.22 to 3.70) p = .008] and DM [odds ratio = 1.74 (95%CI = 1.02 to 2.97) p = .042] were found to be independent predictors for LVSD (Table 2). Age and left main coronary disease almost reached statistical significance.
Table 2. Independent predictors of LVSD.

Discussion
The classic definition of myocardial infarction (MI) by the World Health Organization (WHO) is based on symptoms, electrocardiographic abnormalities, and elevated cardiac enzymes. However, over the past decade the Global MI Task Force has integrated new elements to the definition of MI based on the mechanisms of myocardial injury. Obstructive coronary lesion is the most clinically relevant form of injury and results in troponin release (2,3).
Routine detection of troponin levels using high sensitivity assays that yield a continuous gradient in apparently normal subjects makes it difficult to differentiate myocardial necrosis related to plaque rupture in ACS patients from necrosis in non-ACS patients. Newby et al. discussed the impact of improved test sensitivity on the interpretation of cardiac troponin and emphasized the value of pretest probability when interpreting troponin elevation (3).
The major findings of the present study were: 1) obstructive coronary lesions (flow-limiting) related myocardial injury resulted in greater troponin elevation when compared to other etiologies of myocardial injury, 2) in the context of a flow-limiting coronary artery disease, the degree of troponin elevation implies high-risk features for invasively managed NSTE-ACS patients related to their vascular anatomy, lesion complexity, and the eventual success of percutaneous intervention, and 3) regardless of the mechanism of troponin release, a high level of troponin I was an independent predictor of LVSD in de novo NSTE-ACS patient population.
Troponin I and the presence of hemodynamically significant (flow-limiting) coronary artery disease
Troponin I is independently associated with in-hospital mortality in NSTE-ACS patients. Antman et al. reported that short-term mortality increases with rising levels of cardiac troponin I, and the highest increment in mortality was observed when levels are > 5 ng/ml (4). Additionally, Kleiman et al. (5) demonstrated that invasive management could improve mortality risk in a NSTE-ACS subset of patients with positive cardiac biomarkers.
Interestingly, analyses from the ACTION Registry (NCDR published data) indicate that single vessel flow-limiting coronary artery disease was the most common finding identified by cardiac angiography, and that percutaneous coronary artery intervention was the most common mode of treatment in invasively managed NSTE-ACS patients (6,7). We previously reported that the likelihood of hemodynamically significant coronary artery disease in invasively managed NSTE-ACS patients when the troponin level is more than 5 ng/ml was significantly higher than that in individuals with a lower troponin level (8).
Concern about elevated troponin was reflected in the guidelines that recommend incorporating risk stratification models (TIMI risk score, Grace risk score, or PURSUIT risk model) to the management strategy for NSTE-ACS patients (9). However, none of these models has integrated the additive value of the degree of troponin elevation in their risk-score calculation (10-12).
Being closely associated with mortality and the presence of flow-limiting coronary artery disease, the degree of cardiac troponin elevation should be scored properly in risk stratification modules and contemplated in the timing for invasive management of those presenting with NSTE-ACS.
Troponin I and percutaneous coronary artery interventions in NSTE-ACS
In the setting of ACS, elevated troponin is associated with impaired myocardial tissue perfusion and lower rate of coronary recanalization after percutaneous coronary intervention (13-18). Troponin elevation also signifies adverse short and long-term prognosis in this patient population (19-22). Similarly, in our study, we observed that elevated troponin was associated with increased thrombus burden, worse systolic function, higher complexity of the lesions, and worse post intervention TIMI flow.
Subgroup analysis of ACS clinical trials showed that elevated troponin identified a subset of NSTE-ACS patients who would derive benefit from the addition of antithrombotic therapy and intravenous anti-platelet therapy to a conventional regimen. This is gained via reduction of thrombus formation at the culprit lesion and facilitation of distal micro-thrombi resolution (23-26).
The current guidelines identify the value of elevated troponin when choosing anti-thrombotic therapy, with or without invasive strategy. However, there is no consensus regarding a clinically relevant level of troponin that will provide the most benefit to invasively managed NSTE-ACS patients.
Predictors of left ventricular systolic dysfunction (LVSD) in NSTE-ACS:
Ischemic cardiomyopathy is the main etiology for LVSD in the United States and North America. The development of LVSD following ACS significantly worsens short- and long-term prognosis (17,27,28).
We targeted patients with no prior history of coronary artery disease, heart failure or cardiac surgery who were referred for coronary angiograms for a new diagnosis of NSTE-ACS to assess the predictors of LVSD. Cardiac troponin I levels >5 ng/ml were the most important predictor of LVSD following a new onset NSTE-ACS in patients with no prior history of coronary artery disease (Table 2).
The previous ACC/AHA (2012-2013) guidelines recommended early invasive strategy in NSTE-ACS patients with a systolic ejection fraction of less than 40% (9). The timing of this recommendation was revised in the most recent guidelines (29).
Using clinical characteristics and risk factors at admission to identify risk of heart failure influences therapeutic decisions and permits an individualized approach to each patient. LVSD is a major concern among invasively managed ACS patients and imposes a large economic burden on the health care system. Troponin level could be used as a cost-effective tool to stratify patients who are at risk of LVSD, allowing appropriate early measures to improve their outcome.
Troponin I and Early versus Delayed Intervention in NSTE-ACS
The optimal timing of angiography has not been conclusively established in NTE-ACS (29). In earlier randomized trials, better outcomes were obtained with an early invasive strategy in patient with troponin I elevation when compared to those with normal troponin levels (30). In other reports, investigators found that early invasive intervention was not superior to a delayed invasive approach in NSTE-ACS patients for the prevention of death or myocardial infarction, even in those with positive cardiac biomarkers (31, 32).
The most notable beneficial effect of an invasive versus a conservative strategy in the management of NSTE-ACS patients was demonstrated in the reduction of recurrent MI, although the effect on mortality was seen in high-risk patients only (29,33). Prospective trials to determine a clinically relevant troponin level that will determine the timing of an invasive strategy and its impact on patients’ outcomes are yet to be conducted (1).
Study Limitations
Our cohort study is subject to the standard bias associated with retrospective observations including selection bias, incomplete records, and loss of patients’ long-term follow-up.
The results of our study were derived from a single-center using a particular assay kit for serum troponin testing; thus, generalizability is a concern. Follow-up of cardiac events, recurrent hospitalizations, and long-term adverse events was beyond the scope of our study.
Conclusion
The degree of cardiac troponin I elevation should be incorporated into the risk stratification models of NSTE-ACS to promptly triage high-risk patients to early invasive strategies and tailored anticoagulant therapy to reduce troponin elevation and improve myocardial perfusion.
Acknowledgement
The authors of the study would like to thank Melissa Hodges (Cardiac Clinical Nurse Specialist) for her help with data abstraction.
References
- Abdul Jabbar A. The clinical implications of cardiac troponins. General Med. 2013;1: 108. [CrossRef]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction, J Am Coll Cardiol. 2012 Oct 16;60(16):1581-98. [CrossRef] [PubMed]
- Newby LK, Jesse RL, Babb JD, Christenson RH, De Fer TM, Diamond GA, Fesmire FM, Geraci SA, Gersh BJ, Larsen GC, Kaul S, McKay CR, Philippides GJ, Weintraub WS. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012 Dec 11;60(23):2427-63. [CrossRef] [PubMed]
- Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med.1996;335:1342-9. [CrossRef] [PubMed]
- Kleiman NS, Lakkis N, Cannon CP, Murphy SA, Di Battiste PM, Demopoulos LA, Weintraub WS, Braunwald E. Prospective analysis of creatine kinase muscle-brain fraction and comparison with troponin T to predict cardiac risk and benefit of an invasive strategy in patients with non-ST-elevation acute coronary syndromes. J Am Coll Cardiol. 2002;40:1044-50. [CrossRef] [PubMed]
- Kontos MC, de Lemos JA, Ou FS, et al. Troponin-positive, MB-negative patients with non-ST-elevation myocardial infarction: An undertreated but high-risk patient group: Results from the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network-Get With The Guidelines (NCDR ACTION-GWTG) Registry. Am Heart J. 2010 Nov;160(5):819-25. [CrossRef] [PubMed]
- Chin CT, Wang TY, Li S, et al. Comparison of the prognostic value of peak creatine kinase-mb and troponin levels among patients with acute myocardial infarction: a report from the acute coronary treatment and intervention outcomes network registry–get with the guidelines. Clin Cardiol. 2012;35(7):424-9. [CrossRef] [PubMed]
- Abdul Jabbar A, Ahsan C. Troponin I and the likelihood of hemodynamically significant coronary artery disease in patients with NSTE-ACS. Int J Cardiol. 2013 Dec 5;170(1):e17-9. [CrossRef] [PubMed]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Lincoff AM, Philippides GJ, Zidar JP. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jun 11;127(23):e663-828. [CrossRef] [PubMed]
- Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557–67. [CrossRef] [PubMed]
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non–ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. [CrossRef] [PubMed]
- Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. [CrossRef] [PubMed]
- DeFillipi C, Tocchi M, Parmar R, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long term clinical outcomes. J Am Coll Cardiol. 2000;35:1827–34. [CrossRef] [PubMed]
- Benamer H, Steg PG, Benessiano J, et al. Elevated cardiac troponin I predicts a highrisk angiographic anatomy of the culprit lesion in unstable angina. Am Heart J. 1999;137: 815–20. [CrossRef] [PubMed]
- Wong G, Morrow D, Murphy S, et al. Elevations in troponin T and I are associated with abnormal tissue level perfusion: a TACTICS-TIMI 18 substudy. Circulation. 2002; 106: 202–7. [CrossRef] [PubMed]
- Okamatsu K, Takano M, Sakai S, et al. Elevated troponin T Levels and lesion characteristics in non–ST-elevation acute coronary syndromes. Circulation. 2004; 109: 465–70. [CrossRef] [PubMed]
- Lindahl B, Diderholm E, Lagerqvist B, et al. Mechanisms behind the prognostic value of troponin T in unstable coronary artery disease: a FRISC II substudy. J Am Coll Cardiol. 2001;38:979–86. [CrossRef] [PubMed]
- Heeschen C, van Den Brand M, Hamm C, et al. Angiographic findings in patients with refractory unstable angina according to troponin T status. Circulation. 1999;100: 1509–14. [CrossRef] [PubMed]
- Matetzky S, Sharir T, Domingo M, et al. Elevated troponin I level on admission is associated with adverse outcomeof primary angioplasty in acute myocardial infarction. Circulation. 2000;102:1611–6. [CrossRef] [PubMed]
- Stubbs P, Collinson P, Moseley D, Greenwood T, Noble M. Prognostic significance of admission troponin T concentrations in patients withmyocardial infarction. Circulation. 1996;94:1291–7. [CrossRef] [PubMed]
- Giannitsis E, Muller-Bardorff M, Lehrke S, et al. Admission troponin T level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after primary percutaneous intervention for acute ST segment elevation myocardial infarction. Circulation. 2001;104:630–5. [CrossRef] [PubMed]
- Kontos MC, Shah R, Fritz LM, et al. Implication of different cardiac troponin I levels for clinical outcomes and prognosis of acute chest pain patients. J Am Coll Cardiol. 2004;43:958–65. [CrossRef] [PubMed]
- Morrow DA, Antman EM, Tanasijevic M, et al. Cardiac troponin I for stratification of early outcomes and the efficacy of enoxaparin in unstable angina: a TIMI 11B substudy. J Am Coll Cardiol. 2000;36:1812–7. [CrossRef] [PubMed]
- Lindahl B, Venge P, Wallentin L; for the Fragmin in Unstable Coronary Artery Disease (FRISC) Study Group. Troponin T identifies patients with unstable coronary artery disease who benefit from long-term antithrombotic protection. J Am Coll Cardiol. 1997;29:43–8. [CrossRef] [PubMed]
- Hamm CW, Heeschen C, Goldmann B, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. N Engl J Med. 1999; 340:1623–9. [CrossRef] [PubMed]
- Heeschen C, Hamm CW, Goldmann B, Deu A, Langenbrink L, White HD. Troponin concentrations for stratification of patients with acute coronary syndromes in relation to therapeutic efficacy of tirofiban. PRISM Study Investigators. Platelet Receptor Inhibition in Ischemic Syndrome Management. Lancet. 1999 Nov 20;354(9192):1757-62. [CrossRef] [PubMed]
- St. John Sutton M, Pfeffer MA, Plappert T, et al. Quantitative two-dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation. 1994;89:68 –75. [CrossRef] [PubMed]
- Rao AC, Collinson PO, Canepa-Anson R, Joseph SP. Troponin T measurement after myocardial infarction can identify left ventricular ejection of less than 40%. Heart. 1998;80:223–5. [CrossRef] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, Casey Jr DE, Ganiats TG, Holmes Jr DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Dec 23;64(24):e139-228. [CrossRef] [PubMed]
- Morrow DA, Cannon CP, Rifai N, et al. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA. 2001;286:2405–12. [CrossRef] [PubMed]
- de Winter RJ, Windhausen F, Cornel JH, Dunselman PH, Janus CL, Bendermacher PE, Michels HR, Sanders GT, Tijssen JG, Verheugt FW; Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) Investigators, Early invasive versus selectively invasive management for acute coronary syndromes. N Engl J Med. 2005;353:1095-104. [CrossRef] [PubMed]
- Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, Afzal R, Chrolavicius S, Jolly SS, Widimsky P, Avezum A, Rupprecht HJ, Zhu J, Col J, Natarajan MK, Horsman C, Fox KA, Yusuf S; TIMACS Investigators, Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360:2165-75. [CrossRef] [PubMed]
- Fox KA, Poole-Wilson P, Clayton TC, et al. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet. 2005;366:914-20. [CrossRef] [PubMed]
Cite as: Abdul Jabbar A, Mufti O, Altabaqchali S, Ahsan C, Hasan M, Markert R, White B, Broderick G. High-sensitivity troponin i and the risk of flow limiting coronary artery disease in non-ST elevation acute coronary syndrome (NSTE-ACS). Southwest J Pulm Crit Care. 2017;14(6):296-307. doi: https://doi.org/10.13175/swjpcc059-17 PDF
June 2017 Critical Care Case of the Month
Stephanie Fountain, MD
Pulmonary and Critical Care Medicine
Banner University Medical Center Phoenix
Phoenix, AZ USA
History of Present Illness
The patient is a 60-year-old woman who presented with a month long history of of odynophagia with retrosternal pain and occasional nausea and vomiting.
Past Medical History, Social History and Family History
She has a past medical history of mixed connective tissue disease with anti-phosopholipid antibody. There is also a history of leukocytoclastic vasculitis, chronic leg ulcers, and poor dentition. She also has a history of chronic obstructive lung disease (COPD) and is a current smoker having accumulated about 50 pack-years of cigarette smoking.
Current Medications
- Prednisone 20 mg daily
- Azathioprine 75 mg daily
- Plaquenil 400 mg daily
- Salmeterol/fluticasone BID
- Albuterol prn
Electrocardiographic, Radiologic and Laboratory Evaluation
Her electrocardiogram and chest x-ray were unremarkable. Complete blood count showed a white blood cell count of 10,500 cells per microliter (mcL), hemoglobin 10.3 grams/deciliter (dL), hematocrit 31%, and platelet count of 48,000 cells per mcL. Electrolytes were unremarkable and creatinine was 0.6 mg/dL.
What should be done next? (Click on the correct answer to proceed to the second of six pages)
- Bronchoscopy
- Gastroenterology consult
- Platelet and red blood cell (RBC) transfusion
- 1 and 3
- All of the above
Cite as: Fountain S. June 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;14(6):262-8. doi: https://doi.org/10.13175/swjpcc061-17 PDF
Clinical Performance of an Interactive Clinical Decision Support System for Assessment of Plasma Lactate in Hospitalized Patients with Organ Dysfunction
Robert A. Raschke, MD MS
Hargobind Khurana, MD
Huw Owen-Reece, MBBS
Robert H. Groves Jr, MD
Steven C. Curry, MD
Mary Martin, PharmD
Brenda Stoffer, RN BSN
Banner University Medical Center Phoenix
Phoenix, AZ USA
Abstract
Purpose: Elevated plasma lactate concentration can be a useful measure of tissue hypo-perfusion in acutely deteriorating patients, focusing attention on the need for urgent resuscitation. But lactate is not always assessed in a timely fashion in patients who have deteriorating vital signs. We hypothesized that an electronic medical record (EMR)-based decision support system could interact with clinicians to prompt assessment of plasma lactate in appropriate clinical situations in order to risk stratify a population of inpatients and identify those who are acutely deteriorating in real-time.
Methods: All adult patients admitted to our hospital over a three month period were monitored by an EMR-based lactate decision support system (lactate DSS) programmed to detect patients exhibiting acute organ dysfunction and engage the clinician in the decision to order a plasma lactate concentration. Inpatient mortality was determined for the five risk categories that this system generated, and chart review was performed on a high-risk subgroup to describe the spectrum of bedside events that triggered the system logic.
Results: The lactate DSS segregated inpatients into five strata with mortality rates of 0.8% (95%CI:0.6-1.0%); 2.7% (95%CI:1.0-4.4%); 7.9% (95%CI: 6.0-10.1%), 13.0% (95%CI: 9.0-17.8%) and 42.1% (95%CI: 32.0-52.4%), achieving a discriminant accuracy of 80% (95%CI:76-84%) by AUROC for predicting inpatient mortality. Classification into the two highest risk strata had a positive predictive value for detecting acute life-threatening clinical events of 54% (95%CI: 41.5-66.5%).
Conclusions: Our lactate decision support system is different than previously-described computerized “early warning systems”, because it engages the clinician in decision-making and incorporates clinical judgment in risk stratification. Our system has favorable operating characteristics for the prediction of inpatient mortality and real-time detection of acute life-threatening deterioration.
Introduction
Over 700,000 deaths occur annually in U.S. hospitals (1). Sepsis accounts directly for 37% and indirectly for 56% of these deaths (2). Other common causes of inpatient mortality such as acute hemorrhage and venous thromboembolism (3) share certain early clinical findings with sepsis, in that they may present with deterioration of vital signs and biochemical variables before life-threatening manifestations become obvious (4). Recognition of these findings provides an opportunity for early intervention, which has been shown to improve mortality (5,6). Studies have shown that failure to rapidly recognize acute clinical deterioration is one of the most common root causes of preventable inpatient mortality (4,8).
Early warning systems (EWSs) are a type of clinical decision support system (CDSS) utilized to provide surveillance of hospitalized patients in order to alert clinicians when a patient has findings associated with acute deterioration (19). These typically monitor for abnormal vital signs or laboratory evidence of organ dysfunction, but have included many other types of clinical and laboratory variables (20-23). Modern EWSs utilize logistic regression to weight up to 36 different independent variables and yield highly stratified risk scores (24-26).
We had previous experience developing a simple EWS that triggered when at least two systemic inflammatory response syndrome (SIRS) criteria plus at least one of 14 acute organ dysfunction (OD) parameters was detected. Although this system references SIRS it was found to be nonspecific for sepsis (27), and was subsequently employed in our healthcare system to identify patients deteriorating in real-time regardless of the cause. Subsequent research showed that our SIRS/OD alert system was triggered during the course of 19% of admissions, and that patients who triggered the alert had an odds ratio of 30.1 (95% CI: 26.1-34.5) for inpatient mortality (28). We hypothesized that this SIRS/OD alert system could be used to identify high risk patients who might be further risk-stratified by obtaining a plasma lactate concentration.
Elevated plasma lactate concentration is a particularly useful biochemical marker of acute decompensation. Hyperlactemia is pathophysiologically associated with acute tissue hypoperfusion, and clinically associated with organ dysfunction and mortality (7-11). Hyperlactemia is also associated with the need for urgent clinical interventions such as transfusion and urgent surgery in trauma patients (13,14), and resuscitation of medical patients with sepsis or other life-threatening illnesses (5,15). Lactate assessment is integral to the definition of sepsis (7,16), and an essential component of the Surviving Sepsis Campaign sepsis resuscitation bundle (6). Lactate assessment is integral to achieving sepsis bundle compliance as defined by the Centers for Medicare and Medicaid Services (CMS), which has mandated participating hospitals to report as a measure of quality of care. However, lactate is only ordered about half the time that it ought to be in patients with severe sepsis and septic shock (17,18). To our knowledge, only one previously reported EWS incorporates lactate assessment (29), but this system passively utilized lactate concentration results obtained on admission from the emergency room and was not used for surveillance during hospitalization.
We sought to use our SIRS/OD alert system to actively trigger lactate assessment to identify patients suffering from sepsis or any other life-threatening disease process requiring immediate intervention during hospitalization. We hypothesized that the resulting “lactate decision support system” (lactate DSS) would provide inpatient mortality risk stratification with high discriminant accuracy, and detect acute life-threatening events with high positive predictive value compared to contemporary EWSs.
A lactate DSS with these favorable characteristics could theoretically be used to guide emergent interventions in an effort to save lives, although it was not our aim at this time to perform an interventional trial. The specific aims of this study were to pilot an interactive lactate DSS in our healthcare system, and to calculate its discriminant accuracy for mortality risk stratification, and its positive predictive value as a real-time early warning system.
Methods
We prospectively studied a cohort of all adult inpatients admitted to Banner-University Medical Center - Phoenix, a 650-bed academic hospital in Phoenix Arizona, during the first quarter of 2014. Our research was part of an ongoing system-level patient safety project and was approved by our Institutional Review Board.
The decision support logic was developed at Banner Health using Discern Expert® (Cerner Corporation, North Kansas City MO, USA). The lactate decision support system (lactate DSS) monitored each patient in our EMR for vital signs and laboratory results consistent with SIRS and organ dysfunction, using criteria derived from the standard definition of sepsis (5-7) (Table 1).
Table 1. Lactate DSS trigger logic

If criteria for SIRS and organ dysfunction overlapped in any eight-hour window, the lactate DSS was triggered to respond. An electronic notification was generated to the patient’s nurse and physician alerting them to the possibility of acute clinical deterioration suggested by SIRS and organ dysfunction, and recommending evaluation and resuscitation if appropriate. Decision support included automatic generation of an order for a STAT plasma lactate if one was not previously ordered by the clinician, interactively prompting the clinician to cancel it if they felt it was unnecessary.
Adult admissions during the three-month study period and subsequent inpatient mortality were enumerated using our hospital’s general financial database: MedSeries4® (Siemens Corporation, Washington DC). Although some patients triggered the lactate DSS multiple times over the course of their hospital stay, only the first trigger event was included in our analysis.
Inpatient mortality rates with ninety-five percent confidence intervals were calculated for each of five subgroups: 1) patients who did not exhibit SIRS and organ dysfunction during their hospitalization and therefore did not trigger a lactate DSS response; 2) patients who triggered a lactate DSS response, for whom a DSS-generated lactate order was cancelled by their clinician; 3) patients who triggered the lactate DSS and had a lactate concentration <2.2 mmol/L (normal for our laboratory); 4) patients who triggered the lactate DSS and had an elevated lactate of 2.2-3.9 mmol/L; and 5) patients who triggered the lactate DSS and had a highly elevated lactate >4.0 mmol/L.
It was our hypothesis that mortality in patients who triggered the lactate DSS logic would be equivalent whether the clinician chose not to cancel a DSS-generated lactate order, or the clinician had already entered a lactate order themselves. Therefore, we classified patients into the subgroups above regardless of whether their lactate order was DSS-generated or entered independently by the clinician. In order to confirm the validity of this hypothesis, the mortality rate of all patients with any lactate concentration result (the sum of groups 3, 4 and 5 above), and mortality rates within each lactate concentration strata, were separately analyzed to determine if mortality depended on the method of lactate order entry.
Stratified likelihood ratios and the area under the receiver operating curve (AUROC) generated using the five subgroups described above were calculated to determine the discriminant accuracy of the lactate DSS for the outcome of inpatient mortality.
A subgroup analysis was performed of all study patients with an elevated lactate >2.2 mmol/L (above the upper limit of normal range at our laboratory) detected by a DSS-generated lactate order during the first six weeks of the study. These patients’ charts were reviewed in order to characterize the acute clinical events that triggered a lactate DSS response in this subgroup of patients. A physician researcher reviewed progress notes, laboratory and microbiology results at the time of system activation, and for 72 hours afterwards to make this determination. Patient were determined to be suffering an acute life-threatening clinical event if a new-onset or rapidly-progressive disease process was present at the time the lactate DSS was triggered that required emergent treatment with any one of the following: >1 L intravenous fluid resuscitation, vasopressor infusion, >2 units of packed red blood cell transfusion, endotracheal intubation, advanced cardiac life support, or emergent surgical intervention. Minor clinical events included any diagnosis that required initiation of treatment not included in the definition of acute life threatening clinical events above. False alerts were said to have occurred when no evidence was found that the patient was clinically deteriorating in temporal relationship to lactate DSS activation, or within 72 hours. The positive predictive value of the system was calculated for the real-time detection of acute life-threatening clinical events. Microsoft Research and VassarStats® on-line statistical software were used for statistical calculations.
Results
8,867 adult patients were admitted during our three-month study period. One hundred and ninety-six of 8867 patients (2.2% 95%CI: 1.9-2.5%) died while in the hospital. Seventy percent (138/196) of these inpatient deaths occurred in the 16% (1400/8867) of patients who triggered a lactate DSS response.
Four hundred seventy-nine of 1400 patients who triggered the lactate DSS already had a clinician-ordered lactate. A DSS-generated order for plasma lactate was entered for the remaining 921 patients, but clinicians cancelled 337 of these. DSS-generated lactate orders were resulted for the remaining 584 patients. These patients were merged with 479 patients who had clinician –ordered lactates for the purposes of further analysis after confirmation that mortality did not depend on how the lactate was ordered (Figure 1).

Figure 1. Stratification of inpatients into five subgroups by the lactate DSS.
Patients who did not trigger the lactate DSS logic (n=7467) had a mortality rate of 0.78% (95%CI: 0.58-0.98). Patients who triggered the lactate DSS and for whom a DSS-generated lactate order was cancelled by the clinician (n=337) had mortality of 2.7% (95%CI: 1.0-4.4%). Patients who triggered the lactate DSS and had a lactate concentration in the normal range (< 2.2 mmol/L; n=721) had mortality of 7.9% (95%CI: 6.0-10.1%), and those with elevated lactates of 2.2-3.9 and >4.0 mmol/L (n=247 and n=95) had mortality rates of 13.0% (95%CI: 9.0-17.8%) and 42.1% (95%CI: 32.0-52.4%) respectively (Figure 2).

Figure 2. Inpatient mortality rates (Y-axis: Percent mortality) with 95% confidence intervals for five subgroups of patients stratified by lactate DSS.
The mortality of patients who triggered a lactate DSS response and for whom a lactate concentration was resulted did not depend on whether the order was DSS-generated or entered by the clinician (13.0% versus 12.1% (P=0.71)). Clinician-entered lactate orders were closely temporally related to the onset of organ dysfunction, preceding lactate DSS triggering by < six hours in 52%, <12 hours in 64%, and <24 hours in 75% of cases. Likelihood ratios for mortality in subgroups of patients with lactates <2.2, 2.2-3.9, and >4.0 mmol/L were 6.1 (95%CI: 5.4-6.9), 11.8 (95%CI: 9.5-14.7), and 32.4 (95%CI: 22.0-47.1) respectively.
Five-strata of mortality risk generated by the lactate DSS yielded an AUROC of 0.80 (95% CI: 0.76-0.84) (Figure 3).

Figure 3. Receiver-operating characteristic curve for mortality risk stratification by the lactate DSS.
Focused chart review was performed on 61 patients who had elevated lactate (>2.2 mmol/L) detected by a DSS-generated lactate order. Thirty-three (54%) were experiencing acute life-threatening clinical events at the time the lactate DSS was triggered. These included 18 episodes of sepsis. Sepsis was due to pneumonia in nine patients, catheter-associated blood stream infection, bowel perforation, cellulitis, ascending cholangitis, endocarditis, liver abscess, cholecystitis, perianal abscess, or an unidentified source. Other acute life threatening clinical events included five cases of acute gastrointestinal hemorrhage, three of acute respiratory failure, and one each of post-operative bleeding, cardiogenic shock, acute liver failure, retroperitoneal bleeding, acute myocardial infarction, subdural hematoma, and cerebral dural sinus thrombosis. Twenty-one (64%) of these events occurred outside the intensive care unit. The positive predictive value of the detection of SIRS, organ dysfunction and elevated lactate by the lactate DSS for acute life-threatening clinical events was 54% (95%CI: 41.5-66.5%).
Ten minor clinical events included anemia, atrial fibrillation, post-op third spacing, transient mild hypotension associated with end stage liver disease, sedation related to narcotics, and dialysis disequilibrium. There were 18 false alerts among patients with SIRS, organ dysfunction and elevated lactate detected by the system. (18/61=29%).
Discussion
Our lactate DSS effectively segregated a population of adult inpatients into five subgroups with increasing inpatient mortality. Clinician engagement was critically important in achieving this result. About a quarter (337/1400) of patients who triggered the lactate DSS (simultaneously exhibited SIRS and organ dysfunction) were doing well enough in their clinician’s opinion that the DSS-generated lactate order was cancelled. Clinicians exercised good judgment in this regard, identifying a subgroup of patients with inpatient mortality rate not significantly higher than the overall mortality of all patients admitted during the study. This supports our decision to incorporate clinician judgment in our risk stratification method.
Approximately half of patients (721/1400) who triggered the lactate DSS turned out to have a normal lactate concentration, yet suffered inpatient mortality ten-times higher than patients who did not trigger the system. This likely represents the independent association between SIRS and organ dysfunction with the risk for mortality (27, 31,32).
One hundred twenty-nine patients over 3 months (14.5 per 1000 patient admissions) triggered the lactate DSS and were found to have an elevated lactate concentration because of a DSS-generated lactate order. These patients had >50% probability of experiencing an acute life-threatening clinical event at the time the lactate DSS was triggered, and subsequently suffered 50% inpatient mortality.
Our lactate DSS is consistent with the new definition of sepsis because it uses organ dysfunction in addition to SIRS criteria (7). As stated in the new definition of sepsis, “Nonspecific SIRS criteria such as pyrexia or neutrophilia will continue to aid in the general diagnosis of infection” (7). Although these criteria are nonspecific, they appear to be relatively sensitive for sepsis (7,27). Our lactate DSS has excellent discriminant accuracy for predicting inpatient mortality (AUROC=0.80). It is comparable to other criteria such SOFA (AUROC = 0.74) and the Logistic Organ Dysfunction System (AUROC=0.75).The five strata into which it segregates patients could further translate into a decision support-guided treatment protocol, directing appropriate real-time interventions such as those proposed in Table 2.
Table 2. Proposed stratified clinical response to lactate DSS.

* Our data indicate that RRT activation would occur about twice a week at our hospital.
Our lactate DSS is different than EWSs because it specifically prompts assessment of plasma lactate in patients exhibiting SIRS and organ dysfunction, rather than simply generating a warning. But a discussion of the operating characteristics of previously reported EWSs is useful for purposes of comparison. A review of 33 EWSs has reported AUROCs ranging from 0.66-0.78 (19). Several more recent EWSs reported AUROCs of 0.81-0.88 (23,24,26,33), but AUROC comparisons are confounded by lack of consensus regarding which clinical outcome to analyze. Authors have variously chosen 24-hour mortality, ICU transfer, and cardiac arrest, among other outcomes (20,23,24). Many EWSs yield highly stratified results, which may increase the AUROC by adding detail to the shape of the ROC curve, but this will not improve clinical discrimination unless each resulting strata has a distinct clinical response. If a EWS is simply used to activate a rapid response team (RRT), the clinically-achievable discriminant accuracy is best described by a polygonal AUROC derived from a single cutoff with two resulting strata (activate the RRT, or do not activate the RRT). This two-strata AUROC will invariably be lower than the highly stratified AUROC that many authors report (23,24,26,33). Our AUROC analysis is based on 5 strata, each of which could reasonably trigger a distinct clinical response (Table 2).
Our lactate decision support system has a positive predictive value (PPV) for acute life-threatening clinical events that is superior to that of our previous “sepsis alert” (27) and to those reported in several reviews of EWSs. One review of 39 EWSs reported PPVs ranging from 13.5-26.1% (34), and another review of 25 systems reported a median PPV of 36.7% with interquartile range 29.3-43.8% (34). PPV was not reported for several of the most elegant and well-studied EWSs (22,23,25,32). From the perspective of bedside clinicians and rapid response team members, the efficiency of an alert system is strongly influenced by the PPV, because a poor PPV translates to frequent false alerts. The PPV is of particularly concern when the pretest probability of the outcome of interest is low, as in the case of inpatient mortality (2% at our hospital). Bayes theory indicates that a test with relatively good AUROC will have a poor PPV if the pretest probability is low enough.
Our study has several limitations. Our sample size is small compared to many contemporary EWS studies. We did not have the resources to perform focused chart reviews on all study patients and therefore had to limit individual case analysis to a subgroup of study patients. Our simple treatment of vital sign abnormalities as markers of SIRS is not as elaborate as in many EWSs. Our study is only hypothesis-generating, whereas several EWSs are well validated (25,32). We cannot provide data on how our alert might change bedside interventions by clinicians. To our knowledge, no study to date has proven that using a computerized decision support system or EWS to trigger rapid clinical intervention actually improves patient outcomes.
Conclusions
We developed an automated decision-support system that prompts assessment of plasma lactate concentration in patients exhibiting SIRS and organ dysfunction. Our lactate decision support system is different than previously-described EWSs because it engages the clinician in decision-making and incorporates clinical judgment into risk stratification. This system has favorable operating characteristics for the prediction of inpatient mortality and for detecting acute life-threatening events in real time. We have proposed a stratified clinical response based on classification of patients into five subgroups by this system that requires further testing, but our current study was not designed to demonstrate a benefit on clinical outcomes. Our lactate DSS has the potential to improve sepsis bundle compliance by helping clinicians appropriately order lactate concentrations in patients deteriorating due to the onset of sepsis – a hypothesis we are currently investigating. It also has potential for easy generalizability, particularly to other healthcare systems that share the same EMR as ours, but requires further refinement and validation.
Author Contributions
All authors were involved in conceptualization, design and implementation of the decision support system described in this manuscript, and in preparation of the manuscript, and all approve of the content of the manuscript and vouch for the validity of the data. We list below additional contributions from several of the authors:
RAR: data analysis and interpretation, main author of initial draft of the manuscript.
HOW: data analysis and interpretation, contribution to discussion/conclusions
HK: directly in charge of design and pilot implementation team for the decision support system, data interpretation, contribution to discussion, conclusions
RHG: data interpretation, contribution to discussion, conclusions
SCC: data analysis and interpretation, contribution to discussion, conclusions. Manuscript editing.
MM: data collection and analysis
BS: data collection and analysis
References
- Center for Disease Control. Trends in inpatient hospital deaths: National Hospital Discharge Survey: 2000:2010. NCHS Data Brief 118; 2013. [PubMed]
- Liu V, Escobar G, Greene JD, Soule J, et al. Hospital deaths in patients with sepsis from two independent cohorts. JAMA. 2014;312:90-92. [CrossRef] [PubMed]
- Nichols L, Chew B. Causes of sudden unexpected death of adult hospital patients. J Hosp Med. 2012;7:706-8. [CrossRef] [PubMed]
- McGloin H, Adam SK, Singer M. Unexpected deaths and referrals to intensive care of patients on general wards. Are some cases potentially avoidable? J R Coll Physicians Lond. 1999;33:255-9. [PubMed]
- Rivers E, Nguyen B, Havstad S, Ressler J, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. NEJM. 2001;345:1368-77. [CrossRef] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, Annane D, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580. [CrossRef] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10. [CrossRef] [PubMed]
- National Patient Safety Agency. Safer care for the acutely ill patient: learning from serious incidents. 2007;Report # PSO/5. Available online at: http://www.nrls.npsa.nhs.uk/resources/?EntryId45=59828 (accessed 5/9/17).
- Gultepe E, Green JP, Nguyen H, Adams J, et al. From vital signs to clinical outcomes for patients with sepsis: a machine learning basis for a clinical decision support system. J Am Med Inform Assoc. 2014;21:315-325. [CrossRef] [PubMed]
- Jansen TC, van Bommel J, Woodward R, Mulder PG, Bakker J. Association between blood lactate levels, sequential organ failure assessment sub-scores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. 2009;37:2369-74. [CrossRef] [PubMed]
- Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221-6. [CrossRef] [PubMed]
- Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37:2827-39. [CrossRef] [PubMed]
- Guyette F, Suffoletto B, Castillo JL, Quintero J, Callaway C, Puyana JC. Prehospital serum lactate as a predictor of outcomes in trauma patients: A retrospective observational study. J Trauma. 2011;70:782–6. [CrossRef] [PubMed]
- Vandromme MJ, Griffin RL, Weinberg JA, Rue LW 3rd, Kerby JD. Lactate is a better predictor than systolic blood pressure for determining blood requirement and mortality: Could prehospital measures improve trauma triage? J Am Coll Surg. 2010;210:861–9. [CrossRef] [PubMed]
- Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SL, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752-61. [CrossRef] [PubMed]
- Levy MM, Fink MP, Marshall JC, Abraham E, et al. International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250. [CrossRef] [PubMed]
- Gao R, Melody T, Daniels DF, Giles S and Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care. 2005;9:R764–R770. [CrossRef] [PubMed]
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010; 36: 222–31. [CrossRef] [PubMed]
- Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted "track and trigger" systems. Resuscitation. 2008;77:170-9. [CrossRef] [PubMed]
- Hodgetts TJ, Kenward G, Vlachonikolis IG, Payne S, Castle N. The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation. 2002;54:125-31. [CrossRef] [PubMed]
- Kho A, Rotz D, Alrahi K, Cardenas W, et al. Utility of commonly captured data from an HER to identify hospitalized patients at risk for clinical deterioration. AMIA 2007 symposium proceedings. 404-8.[CrossRef]
- Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892-9. [CrossRef] [PubMed]
- Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, et al. Early detection of impending physiological deterioration among patients who are not in intensive care: Development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7:388-95. [CrossRef] [PubMed]
- Prytherch DR, Smith GB, Schmidt P, Featherstone PI. ViEWS – towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81:932-7. [CrossRef] [PubMed]
- Bailey TC, Yixin C, Mao Y, Lu C, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8:236-42. [CrossRef] [PubMed]
- Churpek MM, Yuen TC, Winslow C, Robicsek AA, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Resp Crit Care Med. 2014;190:649-55. [CrossRef] [PubMed]
- Raschke RA, Owen-Reece H, Khurana H, Groves RH Jr, et al. Clinical performance of an automated systemic inflammatory response syndrome (SIRS)/organ dysfunction alert: a system-based patient safety project. Southwest J Pulm Crit Care. 2014;9:223-9. [CrossRef]
- Khurana, H, Groves RH, Simons MP, Martin M, Stoffer B, et al. Real-time automated continuous sampling of electronic medical records predicts hospital mortality. Am J Med. 2016 Jul;129(7):688-698.e2. [CrossRef] [PubMed]
- Jo S, Lee JB, Jin YH, Jeong TO, et al. Modified early warning score with rapid lactate level in critically ill medical patients: the ViEWS-L score. Emerg Med J. 2013;30:123-9. [CrossRef] [PubMed]
- Rangel-Frausto MS, Pittet, D, Costigan M, et al. The natural history of the Systemic Inflammatory Response Syndrome (SIRS): A prospective study. JAMA. 1995;273:117-123. [CrossRef] [PubMed]
- Matthew M, Churpek F, Zadravecz J, et al. Incidence and prognostic value of the Systemic Inflammatory Response Syndrome and organ dysfunctions in ward patients. Am J Resp Crit Care Med. 2015;192:958-64. [CrossRef] [PubMed]
- Pittet D, Range-Frausto S, Tarara LN, Lin N, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995;21:302-9. [CrossRef] [PubMed]
- Kellett J, Kim A. Validation of an abbreviated Vitalpac early warning score (ViEWS) in 75,419 consecutive admission to a Canadian regional hospital. Resuscitation. 2012;83:297-302. [CrossRef] [PubMed]
- Smith GB, Prytherch DR, Schmidt PE, Featherstone PI, et al. A review and performance evaluation of single-parameter "track and trigger" systems. Resuscitation. 2008;79:11-21. [CrossRef] [PubMed]
- Gao H, McDonnell A, Harrison DA, Moore T, et al. Systemic review and evaluation of physiological track and trigger warning systems for identifying at-risk patients on the ward. Intensive Care Med. 2007;33:667-79. [CrossRef] [PubMed]
Cite as: Raschke RA, Khurana H, Owen-Reece H, Groves RH Jr, Curry SC, Martin M, Stoffer B. Clinical performance of an interactive clinical decision support system for assessment of plasma lactate in hospitalized patients with organ dysfunction. Southwest J Pulm Crit Care. 2017;14:241-52. doi: https://doi.org/10.13175/swjpcc058-17 PDF
May 2017 Critical Care Case of the Month
Sapna Bhatia, MD
David Ling, DO
Michel Boivin, MD
Division of Pulmonary, Critical Care and Sleep Medicine
University of New Mexico School of Medicine
Albuquerque, NM USA
History of Present Illness
A 54-year-old Hispanic male who was incarcerated 3 days prior to hospital admission was brought into the emergency room from prison for alcohol related withdrawal seizures.
Physical Examination
Upon arrival to the ER, the patient was noted to be hypoxic with copious thick secretions in his mouth. He was intubated for airway protection, started on propofol and fentanyl drips as well as intravenous thiamine and folic acid.
Radiography
A chest radiograph was performed (Figure 1).

Figure 1. Portable anterior-posterior (AP) radiograph of the chest.
Which of the following are true regarding management of this patient?
- Phenytoin should be administered for prevention of seizures
- Prophylactic antibiotics for aspiration pneumonia should be administered
- Thiamine and folic acid should be administered
- 1 and 3
- All of the above
Cite as: Bhatia S, Ling D, Boivin M. May 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;14(5):192-8. doi: https://doi.org/10.13175/swjpcc051-17 PDF
Management of Life Threatening Post-Partum Hemorrhage with HBOC-201 in a Jehovah’s Witness
Andrea Mytinger, DO1
Elyce Sheehan, MD1
Nathan Blue, MD2
Kendall P. Crookston, MD, PhD3
Ali I. Saeed, MD 4
1Department of Internal Medicine, 2Department of Maternal and Fetal Medicine, 3Departments of Pathology of Transfusion Medicine, and 4Divisions of Pulmonary, Sleep and Critical Care Medicine
University of New Mexico School of Medicine
Albuquerque, NM USA
Abstract
Background: Post-partum hemorrhage remains the leading cause of maternal mortality worldwide. The obstetrician and critical care physician should be aware of local alternative treatment options for symptomatic anemia secondary to post-partum hemorrhage in patients who cannot receive red blood cell transfusion. Transfusion may not be an option due to strong personal belief, lack of compatible blood, or blood shortage.
Case: A 21-year-old woman, gravida 1 para 1001, was transferred to a tertiary care center for management of severe post-partum hemorrhage (hemoglobin 4.2 g/dL). She had undergone emergent dilation and curettage followed by Bakri tamponade balloon placement at an outside facility. As a member of the Jehovah’s Witness faith, she refused red blood cell transfusion. HBOC-201, a bovine hemoglobin based oxygen carrier, was successfully used to reverse symptomatic, life-threatening anemia.
Conclusion: HBOC-201 can act as a means to reverse severe end-organ damage for patients with severe post-partum hemorrhage and should be considered when no other treatment options are available.
Teaching Points
- HBOC-210 (Hemopure®) is a bovine hemoglobin-based oxygen carrier (HBOC), used as a means to reverse severe anemia in those patients who cannot receive RBC transfusion.
- The current generation of HBOCs carry fewer side effects than their predecessors. Common side effects include transient hypertension, abdominal complaints, jaundice, elevated liver and pancreatic enzymes and decreased urine output.
- The teratogenic effects of HBOC-201 remains unknown in humans.
Introduction
Worldwide, there are an estimated 14 million pregnancy-related hemorrhages each year and 25% of maternal mortality can be attributed to post-partum hemorrhage (1). While the majority of post-partum hemorrhage leading to acute blood loss anemia is treated with red blood cell (RBC) transfusion, there remains a significant subset of patients who are unable to receive this life-saving modality. In instances where RBC transfusion is not an option due to lack of compatible blood, blood shortage, or strong patient personal beliefs, there remain alternative options for management. We report the case of a young Jehovah’s Witness who presented with symptomatic anemia secondary to severe post-partum hemorrhage, treated successfully with an experimental protocol using HBOC-201 (Hemopure®).
Case
A 21-year-old female, gravida 1 para 1001, Jehovah’s Witness was transferred to a tertiary care hospital for management of post-partum hemorrhage after spontaneous vaginal delivery at 40 weeks of gestation. The patient received 3 boluses of intravenous oxytocin, 800mcg of misoprostol and 1 dose of intramuscular (IM) carboprost trimethamine. She then underwent a dilation and curettage for presumed retained products of conception. A Bakri tamponade balloon catheter (Figure 1) was placed vaginally and the patient was transferred for a higher level of care.

Figure 1. An example illustration of the Bakri vaginal tamponade balloon, placed in the uterus in attempt to apply pressure to bleeding vessels.
Prior to delivery, the patient’s hemoglobin (Hb) and hematocrit (Hct) were initially 12.4 g/dL and 36.4 %, respectively, which decreased to 5.5 g/dL and 16.4%.
On arrival, the patient ‘s heart rate was 148 beats per minute while on 2L of oxygen. The blood pressure was 93/36 mmHg. She was pale and tired-appearing with conjunctival pallor. Her abdomen exhibited generalized mild tenderness to palpation. The Bakri balloon was in place with 100 mL of drainage noted. Laboratory results revealed Hb of 4.2 g/dL, lactate of 1.2 mmol/L and troponin I of 1.820 ng/mL. The patient refused transfusion of RBC, and the other major blood products, citing her faith. After a prolonged discussion, the patient consented to the use of HBOC-201.
The patient received 2 units of HBOC-201 along with 1,000 mg of ascorbic acid. Her Hb increased from 4.2 g/dL to 4.8 g/dL, much less than the anticipated 1 g/dL increase with each unit of HBOC-201 raising concern for ongoing hemorrhage. An ultrasound was performed which revealed ongoing bleeding from the lower uterine segment behind the balloon. Methylergonovine 0.2mg IM every 6 hours was started and the patient received two additional units of HBOC-201 however, during infusion of the second unit her oxygen requirement increased from 2L by nasal cannula to 15L high flow mask at 80% FiO2. The transfusion was stopped and a chest radiograph revealed diffuse parenchymal opacities with prominent interstitial markings and small bilateral pleural effusions suggestive if fluid overload / pulmonary edema. The patient then underwent gel foam uterine artery embolization by Interventional Radiology for definitive management.
On hospital day 2 the patient’s heart rate was 114 beats per minute and Hb was 5.2 g/dL, prompting infusion of an additional 2 units of HBOC-201. Due to continued hypoxia and radiographic evidence of fluid overload, diuretic therapy was administered. Her oxygen requirement decreased to 5L by nasal cannula, however did not improve from there despite a negative fluid balance, so a CT scan of the chest was performed which revealed significant bilateral basal atelectasis. The patient’s oxygen requirement resolved with incentive spirometry. The Bakri vaginal balloon was removed and minimal bleeding was observed.
On hospital day 3 the patient’s blood pressure increased to 176/78 mmHg. This, in the setting of proteinuria, peripheral edema and elevated aspartate aminotransferase (AST) to 104 unit/L (6-58 Unit/L) raised a suspicion for post-partum preeclampsia with severe features. Intravenous magnesium sulfate was briefly initiated for seizure prophylaxis, however it was discontinued after her blood pressure stabilized and the hypertension was attributed to a possible side effect of HBOC-201.
The patient received a total of 7.5 units of HBOC-201 over the course of 4 days in the MICU. Her troponin peaked on hospital day 2 at 2.930 ng/mL, and continued to downtrend with multiple infusions of HBOC-201. The patient’s own hematocrit began rising on hospital day 5 (Figure 2).

Figure 2. Illustration of the hemoglobin and hematocrit over the course of the patient’s hospitalization and at her first out-patient follow up visit. The arrows indicate when HBOC-201 was infused. Troponin I is also depicted on this graph to illustrate the resolution of severe end-organ damage due to the severe anemia.
The patient was transferred to the obstetrics floor on hospital day 7. In accordance with recent post-partum hemorrhage recommendations, she received 1025 mg of IV iron dextran. She was discharged home in stable condition on hospital day 8 with a Hb of 6.7 g/dL and Hct of 21%. Outpatient follow-up revealed significant improvement in anemia with a Hb of 9.2 g/dL and Hct of 30% one week after discharge.
Discussion
Acute post-partum hemorrhage leading to severe anemia remains the leading cause of maternal death worldwide (2). While the majority of post-partum hemorrhage leading to acute blood loss anemia is treated with transfusion of packed RBC or other blood products, there are certain subsets of patients who are unable to accept these products. This case demonstrates the use of a bovine hemoglobin-based oxygen carrier in a Jehovah’s Witness patient with severe post-partum hemorrhage who refused blood products. There have been multiple case reports regarding the use of HBOC-201 in severely anemic Jehovah’s Witness patients; however, there is no published report to our knowledge on the use of HBOC-201 in patients with symptomatic post-partum hemorrhage.
Hemoglobin-based oxygen carriers were developed in response to the infectious issues associated with donor RBC and in an attempt to come up with an alternative treatment in those situations where RBC transfusion was not an option. The first generation of these products was known to cause renal toxicity and coagulopathy (3,4). HBOC-201 is a second generation HBOC that is a cell-free, stroma-free, polymerized version of bovine hemoglobin. Because it contains no cell membrane, it is compatible with all blood types (no cross matching is needed). The shelf life is 36 months at room temperature (5) (no refrigeration or sophisticated supply network is needed). A number of randomized control trials have been done to evaluate HBOC-201 (and other similar products) as a potential RBC replacement. However, after infusion the short 24-hour half-life and statistical increase in adverse events associated with administration made it apparent that these HBOCs were not interchangeable with RBC for routine transfusion. While they are not interchangeable, many clinicians feel that the risk-benefit profile is favorable in severely anemic patients who cannot receive RBC. HBOC-201 is not yet approved for use in the United States, and therefore cannot be used outside of clinical trials. Several compassionate use studies are available in the United States to treat patients with life-threatening anemia when no other treatment option is available. Worldwide only a few countries have approved the use of HBOC-201 (6).
The side effect profile of the second generation HBOC’s is much preferable to that of the first (4). Reported class effects of HBOC use include hypertension, esophageal dysmotility and increased risk for myocardial infarction, all of which are related to vasoconstriction secondary to increased nitric oxide scavenging in these products (5). HBOC-201 in particular, has not been reported to increase risk of myocardial infarction. Rather, it has been reported that HBOC-201 reduces cardiac hypoxia in the setting of severe anemia (7). Mongan et al. (8) found that, while HBOC-201 causes transient systemic and pulmonary hypertension in swine, blood flow to 8 major organs, including the heart, was unchanged compared to controls. Serruys et al. (9) found no significant change in coronary blood flow and no vasoconstriction in humans pre-oxygenated with HBOC-201 prior to Percutaneous Coronary Intervention for coronary artery disease. In this case, the patient presented with troponinemia, indicating type 2 demand ischemia in the setting of severe anemia. Troponin levels began to down-trend after HBOC-201 infusion.
Common side effects of HBOC-201 in particular include transient hypertension, abdominal complaints, jaundice, elevated liver and pancreatic enzymes (10) and bovine methemoglobinemia (11). To prevent the increased oxidation of infused HBOC-201 to methemoglobin, ascorbic acid is co-administered; methemoglobin levels should be monitored and treated with methylene blue should they become significantly elevated (5).
This patient did experience increased hypoxia while receiving a unit of HBOC-201 which resulted in concern for transfusion reaction and transient discontinuation of the HBOC-201 infusion. It must be noted that HBOC-201 contains no cellular or plasma components, thus many transfusion reactions such as Transfusion Related Acute Lung Injury (TRALI) are an impossibility. HBOC-201 has been associated with volume overload; as it is a colloid this is a known complication (12). Volume overload was suspected, however, the patient did not improve with diuresis, and a chest CT revealed profound atelectasis. Given that her hypoxia greatly improved with incentive spirometry and ambulation, this was deemed unlikely to be a reaction associated with HBOC-201, but rather related to being bed-bound and critically ill.
One unit of HBOC-201 will raise serum Hb from 0.5g/dL to 2g/dL (12). One to two units of HBOC-201 are typically given for Hb levels <6 g/Dl, with additional units provided to maintain a goal Hemoglobin greater than 6g/dL (11, 12). With a half-life of 19-24 hours (5, 13), HBOC-201 must be infused regularly until the patient’s bone marrow production of RBC is sufficient, as evidenced by increases in hematocrit. It should be noted that HBOC-201 will only increase serum hemoglobin and not hematocrit; an initial decrease in hematocrit may be seen after infusion secondary to hemodilution (12).
The patient presented above experienced both transient hypertension and an increase in her serum AST, raising concern for post-partum preeclampsia. She was started on treatment for severe preeclampsia, however these affects were later attributed to the HBOC-201.
HBOC-201 is currently not recommended during pregnancy. One animal study in rats indicated that HBOC-201 infusion during organogenesis resulted in decreased litter size and increased incidence of external fetal malformations. This was thought to be related to decreased function of an inverted yolk sac, the primary nutritive organ for rat pups in utero (14). Holson et al. (15) performed a similar study on dogs which did not reveal a statistically significant difference in fetal malformations or other study end-points when compared to control. Canines and humans do not have an inverted yolk sac. Thus, it has been hypothesized that teratogenic effects of HBOC-201 do not apply to humans, however, more studies are needed. At least one US expanded access study allows pregnant women with the potential of massive blood loss (e.g. those with placenta accreta, placenta percreta) to consent to the study while still pregnant. However, HBOC-201 cannot be given until after delivery.
HBOC-201 in this case was utilized as a means to reverse severe end-organ damage due to anemia. This Jehovah’s Witness patient refused blood products, citing religious beliefs. Jehovah’s Witnesses in general will not receive “primary” blood components which include red blood cells, platelets and plasma. Other components, including albumin, clotting factors and HBOCs are considered “conscience items” through the church, where-in the individual can decide for themselves if they wish to receive them (5). With an estimated 1.2 million Jehovah’s Witnesses in the United States alone, alternative treatment options for this patient population are imperative (5).
While transfusion of allogeneic blood products remains the standard of care for treatment of severe post-partum hemorrhage, there are certain situations where this is not available. These might include lack of resources in a rural setting, blood product shortages, and inability to cross-match blood products given patient antibodies or patient denial of blood products due to personal or religious beliefs. HBOC’s are currently not approved for use in the United States, however they can be used on a limited compassionate use basis with FDA IND and local IRB approval, either as part of a planned expanded use study or on an emergency approval basis. Referral to a center with an expanded use protocol should be considered for a woman with the potential for massive bleeding who cannot receive RBC.
References
- Enakpene CA, Morhason-Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post-partum hemorrhage during third stage of labor. J Obstet Gynaecol Res. 2007 Dec;33(6):810-7. [CrossRef] [PubMed]
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014 2(6):e323–e333.[CrossRef] [PubMed]
- Creteur J, Vincent JL. Hemoglobin solutions. Crit Care Med. 2003 Dec;31(12 Suppl):S698-707. [CrossRef] [PubMed]
- Marinaro J, Smith J, Tawil I, Billstrand M, Crookston KP. HBOC-201 use in traumatic brain injury: case report and review of literature. Transfusion. 2009 Oct;49(10):2054-9. [CrossRef] [PubMed]
- Epperla N, Strouse C, VanSandt AM, Foy P. Difficult to swallow: warm autoimmune hemolytic anemia in a Jehovah's Witness treated with hemoglobin concentrate complicated by achalasia. Transfusion. 2016 Jul;56(7):1801-6. [CrossRef] [PubMed]
- Greenburg AG, Kim HW. Hemoglobin-based oxygen carriers. Crit Care. 2004;8 Suppl 2:S61-4. [CrossRef] [PubMed]
- Fitzgerald MC, Chan JY, Ross AW, Liew SM, Butt WW, Baguley D, et al. A synthetic haemoglobin-based oxygen carrier and the reversal of cardiac hypoxia secondary to severe anaemia following trauma. Med J Aust. 2011 May;194(9):471-3. [PubMed]
- Mongan PD, Moon-Massat PF, Rentko V, Mihok S, Dragovich A, Sharma P. Regional blood flow after serial normovolemic exchange transfusion with HBOC-201 (Hemopure®) in anesthetized swine. J Trauma. 2009 Jul;67(1):51-60. [CrossRef] [PubMed]
- Serruys PW, Vranckx P, Slagboom T, Regar E, Meliga E, de Winter RJ, et al. Haemodynamic effects, safety, and tolerability of haemoglobin-based oxygen carrier-201 in patients undergoing PCI for CAD. EuroIntervention. 2008 Mar;3(5):600-9. [CrossRef] [PubMed]
- Van Hemelrijck J, Levien LJ, Veeckman L, Pitman A, Zafirelis Z, Standl T. A safety and efficacy evaluation of hemoglobin-based oxygen carrier HBOC-201 in a randomized, multicenter red blood cell controlled trial in noncardiac surgery patients. Anesth Analg. 2014 Oct;119(4):766-76. [CrossRef] [PubMed]
- Jordan SD, Alexander E. Bovine hemoglobin: a nontraditional approach to the management of acute anemia in a Jehovah's Witness patient with autoimmune hemolytic anemia. J Pharm Pract. 2013 Jun;26(3):257-60. [CrossRef] [PubMed]
- Mer M, Hodgson E, Wallis L, Jacobson B, Levien L, Snyman J, et al. Hemoglobin glutamer-250 (bovine) in South Africa: consensus usage guidelines from clinician experts who have treated patients. Transfusion. 2016 Sep. [CrossRef] [PubMed]
- Donahue LL, Shapira I, Shander A, Kolitz J, Allen S, Greenburg G. Management of acute anemia in a Jehovah's Witness patient with acute lymphoblastic leukemia with polymerized bovine hemoglobin-based oxygen carrier: a case report and review of literature. Transfusion. 2010 Jul;50(7):1561-7. [CrossRef] [PubMed]
- Stump DG, Holson JF, Harris C, Pearce LB, Watson RE, DeSesso JM. Developmental toxicity in rats of a hemoglobin-based oxygen carrier results from impeded function of the inverted visceral yolk sac. Reprod Toxicol. 2015 Apr;52:108-17. [CrossRef] [PubMed]
- Holson JF, Stump DG, Pearce LB, Watson RE, DeSesso JM. Absence of developmental toxicity in a canine model after infusion of a hemoglobin-based oxygen carrier: Implications for risk assessment. Reprod Toxicol. 2015 Apr;52:101-7. [CrossRef] [PubMed]
Cite as: Mytinger A, Sheehan E, Blue N, Crookston KP, Saeed AI. Management of life threatening post-partum hemorrhage with HBOC-201 in a Jehovah’s witness. Southwest J Pulm Crit Care. 2017;14(4):177-84. doi: https://doi.org/10.13175/swjpcc031-17 PDF
Tracheal Stoma Necrosis: A Case Report
Stella Pak, MD
Arjan Flora, MD
Young-Sook Yoon, MD
Department of Medicine
University of Toledo Medical Center
Toledo, OH, USA
Abstract
Acute tracheal dilatation, due to an overinflated cuff, has been reported early in the course of mechanical ventilation through an endotracheal tube. Tracheal stoma necrosis is a rare complication, but such can accompany acute tracheal dilation. Herein, we report a case of tracheal necrosis 9 days following tracheostomy placement in a 71-year old woman associated with overinflation of the tracheal tube cuff. This case report aims to 1) add to the scant body of knowledge about the diagnosis and management for the patients with tracheal stoma necrosis and 2) raise awareness for error-traps in interpreting diagnostic images, specifically satisfaction of search error, inattentional blindness error, and alliterative error.
Case Report
A 71-year-old woman with a history of chronic respiratory failure on mechanical ventilation presented to the emergency department for bleeding around the tracheostomy site. The tracheostomy was recently inserted 9 days prior to admission. A chest radiograph demonstrated left lower lobe atelectasis, pleural effusion, and cardiomegaly that was consistent with pre-existing congestive heart failure (Figure 1).

Figure 1. Chest radiograph (AP) performed during first admission.
The cuff overinflation was demonstrated as a spherical shaped hypolucent region surrounding the trachea. However, the lesion escaped attention possibly because the focus of attention was limited to the thoracic compartment. A CT of the soft tissue in the neck ruled out the possibility of hematoma or infection. However, the features suggestive of overinflation of tracheostomy tube once again escaped attention. The spherical shaped hypolucent area, representing the cuff, was 3.9 cm in the anterior-posterior axis and 3.8 cm along the right-left axis.
A fiberoptic bronchoscopy through the tracheostomy tube revealed a large blood clot obstructing the distal end of the tube. A necrotic lesion around the stoma was also found. Careful observation via the bronchoscope during the procedure revealed no tearing or rupture. The patient was conservatively treated with vancomycin and cefepime for treatment of a ventilator-associated pneumonia. The oozing of blood from the tracheostomy stopped on with conservative wound care, including cleaning and dressing. She returned back to her baseline and was subsequently discharged on 3rd day of admission. During this first admission, a tracheostomy tube exchange was not done due to bleeding from the stoma.
The patient was readmitted 12 days after discharge for an episode of hematemesis of approximately 400 mL of bright red blood. A chest radiograph showed satisfactory position of tracheotomy tube and cardiomegaly at baseline (Figure 2).

Figure 2. Chest radiograph (AP) after readmission.
For the third time, the features suggestive of cuff-overinflation went unnoticed, delaying accurate diagnosis and proper treatment.
As a part of the patient’s evaluation, a CT of the chest with intravenous contrast was done, revealing the overinflated cuff of the trachea tube into the soft tissue of the neck (Figure 3).

Figure 3. Thoracic CT scan showing the overinflated tracheostomy cuff in the (A) coronal, (B) sagittal, and (C) axial views.
The ovoid shaped hypolucent area, representing the cuff, was 5.3 cm in the anterior-posterior axis and 4.6 cm along the right-left axis.
The Shiley proximal tracheal tube was urgently replaced with a portex Bivona tracheal tube. The new tracheostomy tube is more extensible, soft, and longer in distal length. Postoperatively, the patient was kept ventilated in the ICU. Repeated chest CT showed the new tracheostomy tube in satisfactory position and normalization of trachea shape. She made an uneventful recovery and was discharged 8 days after the tracheotomy tube replacement.
Discussion
A case of nonfatal hemorrhage due to innominate artery erosion with soft tissue necrosis at the stoma site of a tracheostomy is presented. In this ventilator-dependent patient with a recent tracheotomy stoma creation, an overinflated cuff of a tracheotomy tube was the key culprit in the pathology. Tracheal tube cuff pressure should be monitored so that it does not exceed a reasonable estimate of capillary perfusion pressure. Cuffs with pressure over 25 mmHg can compress the surrounding soft tissue, including delicate vascular structures. The damage to the vasculature in contact with the tube can result in ischemic necrosis in the soft tissue. If left untreated, these necrotic regions can develop infection or undergo fibrosis, leading to progressive stenosis (1).
A number of cognitive errors led to multiple episodes of misdiagnosis in this patient. Satisfaction of search error is a type of false negative error caused by premature termination of search after an abnormality has been detected (2). In this patient, we readily detected several abnormalities—cardiomegaly, pulmonary atelectasis, and pleural effusion. These initial findings likely led us to subconsciously neglect later findings.
Inattentional blindness error is a false negative error caused by the psychological lack of attention on an unexpected stimulus (3). In the present case, none of the diagnostic imaging was taken to check for cuff-overinflation. The images from the first admission were ordered for a concern of NG tube malposition, infection, and hematoma. The images ordered during the second admission were ordered to check the tracheotomy tube position. The thoracic compartment (the area for the expected abnormalities) received a disproportionately large amount of attention, whereas only a scant amount of attention was paid to the neck compartment.
Alliterative error is an error caused by a preconceived notion from a previous interpretation by a colleague or oneself (4). The negative finding in the previous reports could have affected the subsequent interpretative performance.
To the best of our knowledge, there are only 3 other cases of soft tissue necrosis caused by cuff overinflation. In two of these cases, the extended trachea did not recoil back to the previous size (5, 6). In the presented case, the stretched trachea recoiled back, similar to the case described by Sachdeva and his colleagues (7). The prognostic value of this difference in recovery is unknown, but might have a significant clinical implication. To explore the clinical relevance of this finding, more data on this condition is needed.
Teaching Points
- Careful attention should be paid to cuff inflation pressure in patients presenting with bleeding at the tracheostomy site.
- Conscious efforts to avoid well-known errors in diagnostic image interpretation, such as satisfaction of search error, and inattentional blindness error, should be made to improve diagnostic accuracy.
References
- De Leyn P, Bedert L, Delcroix M, et al. Tracheotomy: clinical review and guidelines. Eur J Cardiothorac Surg. 2007 Sep;32(3):412-21. [CrossRef] [PubMed]
- Ashman CJ, Yu JS, Wolfman D. Satisfaction of search in osteoradiology. AJR Am J Roentgenol. 2000 Aug;175(2):541-4. [CrossRef] [PubMed]
- Richards A, Hannon EM, Derakshan N. Predicting and manipulating the incidence of inattentional blindness. Psychol Res. 2010 Nov;74(6):513-23. [CrossRef] [PubMed]
- Berlin L. Malpractice issues in radiology. Alliterative errors. AJR Am J Roentgenol. 2000 Apr;174(4):925-31. [CrossRef] [PubMed]
- Rhodes A, Lamb FJ, Grounds RM, Bennett ED. Tracheal dilatation complicating tracheal intubation. Anaesthesia. 1997 Jan;52(1):70-2. [CrossRef] [PubMed]
- Honig EG, Francis PB. Persistent tracheal dilatation: onset after brief mechanical ventilation with a "soft-cuff" endotracheal tube. South Med J. 1979 Apr;72(4):487-90. [CrossRef] [PubMed]
- Sachdeva A, Pickering EM, Reed RM, Shanholtz CB. Ice cream cone sign: reversible ballooning of the trachea due to tracheostomy tube cuff overinflation. BMJ Case Rep. 2016 May 4;2016. [CrossRef] [PubMed]
Cite as: Pak S, Flora A, Yoon Y-S. Tracheal stoma necrosis: a case report. Southwest J Pulm Crit Care. 2017;14(4):172-6. doi: https://doi.org/10.13175/swjpcc032-17 PDF
April 2017 Critical Care Case of the Month
Robert A. Raschke, MD
Banner University Medical Center-Phoenix
Phoenix, AZ USA
History of Present Illness
A 20-year-old woman was transferred from another medical center for care. She was pregnant and initially presented with a one day history of crampy abdominal pain with nausea and vomiting after eating old, bad tasting chicken two days previously. She had pain of her right arm and a non-displaced humeral fracture was seen on x-ray. The etiology of the fracture was unclear. Her illness rapidly progressed to respiratory distress requiring intubation. The fetus had deceleration of heart tones leading to a cesarean section and delivery of a non-viable infant. Subsequently, she had rapid progression of shock and anuria.
Past Medical History
She had a previous history of a seizure disorder which was managed with levetiracetam, clonazepam, and folic acid. There was a previous intentional opiate overdose 2 years earlier. One month prior to admission she had visited her husband in Iraq. After returning to the US 3 weeks prior to admission, she developed a sore throat and was treated with penicillin. She smokes tobacco hookah and marijuana. There is a positive family history of gout.
Physical Examination
- Vital signs: heart rate 109, blood pressure 102/78 mm Hg while on norepinephrine, respiratory rate 22, temperature 36.5º C.
- General: She was sedated and intubated. She had a splint on her right arm.
- Lungs: clear anteriorly
- Heart: regular rhythm without murmur
- Abdomen: firm without palpable organomegaly or masses.
- Neurological examination: There was movement of all extremities. Muscle tone was normal. Deep tendon reflexes were normal. Plantar reflexes were down going.
- Skin: diffuse erythematous macular popular rash on the trunk and back (Figure 1).

Figure 1. Photograph of patient’s back showing rash.
Initial Laboratory Evaluation
- CBC: hemoglobin 14.5 gm/dL, platelet count 299,000 cells/mcL, WBC 41,000 cells/mcL, vacuolated polymorphonuclear leukocytes were noted
- Electrolytes: Na+ 135 mmol/L, K+ 4.9 mmol/L, Cl- 95 mmol/L, HCO3- 18 mmol/L
- Renal function: creatinine 3.9 mg/dL, blood urea nitrogen (BUN) 59 mg/dL
- Liver enzymes: AST 294 (normal 8-48 U/L), ALT 303 (normal 7-55 U/L), ALP 187 (normal 45-115 U/L).
- Glucose: 58
Which of the following should be done immediately? (Click on the correct answer to proceed to the second of five pages)
Cite as: Raschke RA. April 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;14(4):134-40. doi: https://doi.org/10.13175/swjpcc039-17 PDF
March 2017 Critical Care Case of the Month
Kyle J. Henry, MD
Banner University Medical Center Phoenix
Phoenix, AZ USA
History of Present Illness
A 50-year-old man presented to the emergency room via private vehicle complaining of 5 days of intermittent chest and right upper quadrant pain. Associated with the pain he had nausea, cough, shortness of breath, lower extremity edema, and palpitations.
Past Medical History, Social History, and Family History
He had a history of hypertension and diabetes mellitus but was on no medications and had not seen a provider in years. He was disabled from his job as a construction worker. He had smoked a pack per day for 30 years. He was a heavy daily ethanol consumer. He had an extensive family history of diabetes.
Physical Examination
- Vitals: T 36.4 C, pulse 106/min and regular, blood pressure 96/69 mm Hg, respiratory rate 19 breaths/min, SpO2 98% on room air
- Lungs: clear
- Heart: regular rhythm without murmur.
- Abdomen: mild RUQ tenderness
- Extremities: No edema noted.
Electrocardiogram
His electrocardiogram is show in Figure 1.

Figure 1. Admission electrocardiogram.
Which of the following are true regarding the electrocardiogram? (Click on the correct answer to proceed to the second of seven pages)
- The lack of Q waves in V2 and V3 excludes an anteroseptal myocardial infarction
- The S1Q3T3 patter is diagnostic of a pulmonary embolism
- There are nonspecific ST and T wave changes
- 1 and 3
- All of the above
Cite as: Henry KJ. March 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;14(3):94-102. doi: https://doi.org/10.13175/swjpcc021-17 PDF
Ultrasound for Critical Care Physicians: Unchain My Heart
William Mansfield, MD
Michel Boivin, MD
Division of Pulmonary, Critical Care and Sleep Medicine
Department of Medicine,
University of New Mexico School of Medicine
Albuquerque, NM USA
A 46-year-old man presented after a motor vehicle collision. He suffered abdominal injuries (liver laceration, avulsed gall bladder) which were successfully managed non-operatively. The patient remained intubated on mechanical ventilation and remained hypotensive after the injuries resolved. The patient required norepinephrine at low doses to maintain a normal blood pressure. It was noted the patient had a history of remote tricuspid valve replacement. A bedside echocardiogram was then performed to determine the etiology of the patient’s persistent hypotension after hypovolemia had been excluded.
Video 1. Apical four chamber view centered on the right heart.
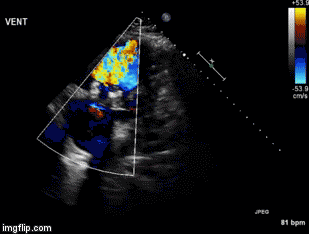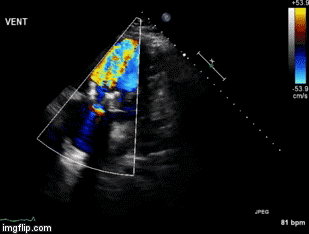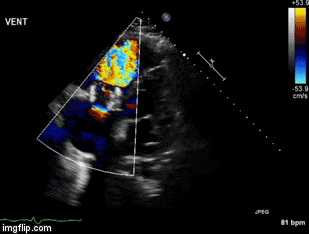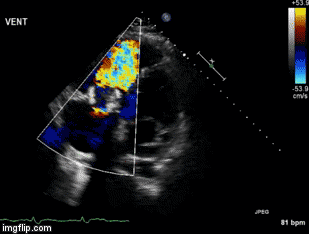
Video 2. Apical four chamber view centered on the right heart, with color Doppler over the right atrium and ventricle.
Video 3. Right ventricular inflow view.
Figure 1. Continuous-wave Doppler tracing through the tricuspid valve.
What tricuspid pathology do the following videos and images demonstrate? (Click on the correct answer to proceed an explanation and discussion)
Cite as: Mansfield W, Boivin M. Ultrasound for critical care physicians: unchain my heart. Southwest J Pulm Crit Care. 2017;14(2):60-4. doi: http://doi.org/10.13175/swjpcc013-17 PDF
February 2017 Critical Care Case of the Month
Morgan Wong, DO
Nicholas Villalobos, MD
Department of Internal Medicine
University of New Mexico
Albuquerque, NM USA
History of Present Illness
A 68-year-old man presented to the emergency department with a one-day history of lower back pain, arthralgias, and malaise. The patient had a previous splenectomy and was concerned about influenza.
Past Medical History, Social History, and Family History
He has a history of osteoarthritis, seasonal allergies, and splenectomy. He is a nonsmoker. Family history is noncontributory.
Physical Examination
Upon admission, the patient’s vital signs were notable for a temperature of 35.3 degrees Celsius, blood pressure of 74/44 mmHg, oxygen saturation of 85% on room air with a respiratory rate of 24 breaths per minute. Physical exam was prominent for non-pitting edema of the distal upper and lower extremities, as well as diffuse macular rash of the palms and soles.
Laboratory
CBC
- White blood cell count of 6.77 X103 cells/uL
- Hemoglobin of 13.8 gm/dL
- Hematocrit of 43.7%
- Platelet count of 19 x 103 /uL
Chemistry
- Creatinine of 3.0 mg/dL
- CO2 < 10 mmol/L
- Anion gap >18 mmol/L
- Liver function tests
- Alanine aminotransferase (ALT) of 511 U/L
- Aspartate aminotransferase (AST) of 529 U/L
- Total bilirubin of 1.0 mg/dL
Coagulation
- INR of 2.07
- Prothromin time of 22.5 seconds
- Partial thromoboplastin time of 82.3 seconds
- Fibrinogen level was 71 mg/dL
Arterial blood gases
- pH of 6.91
- pCO2 54 mmHg
- pO2 263
- HCO3 of 7.7 mmol/L
Procalcitonin >200 ng/ml.
His blood peripheral smear was examined.

Figure 1: Peripheral blood smear on admission.
Given the results of the preliminary laboratory results and peripheral smear what hematologic abnormality are you most concerned with at this time? (Click on the correct answer to proceed to the second of five pages)
- Autoimmune hemolytic anemia (AIHA)
- Disseminated intravascular coagulopathy (DIC)
- Microangiopathic hemolytic anemia (MAHA)
- Thrombotic thrombocytopenic purpura (TTP)
Cite as: Wong M, Villalobos N. February 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;14(2):54-9. doi: https://doi.org/10.13175/swjpcc144-16 PDF
January 2017 Critical Care Case of the Month
Seth Assar, MD
Clement U. Singarajah, MD
Pulmonary and Critical Care Medicine
Banner University Medical Center Phoenix – Phoenix
Phoenix VA Medical Center
Phoenix, AZ USA
History of Present Illness
The patient is a 48-year-old man who presented with two days of progressive shortness of breath and non-productive cough. There were no associated symptoms and the patient specifically denied fever, chills, night sweats, myalgia or other evidence of viral prodrome. He had no chest pain or tightness, nausea, vomiting, or leg swelling and he could lay flat. He had no recent travel or sick contacts and was Influenza-immunized this season.
Past Medical History
- Hypertension
- Hyperlipidemia
- Type 2 diabetes mellitus with a recent hemoglobin A1C of 11%
Social History
- Cook at pizzeria
- Gay and lives at home with roommate of several years
- Smokes marijuana weekly.
- Prior history of cocaine use
Family History
- Noncontributory
Physical Examination
- Vitals: T 99.1º F / HR 125 / BP 193/93 / RR 24 / SpO2 88%
- General: Tachypneic. Alert and oriented X 4.
- Lungs: Crackles at bases bilaterally, no wheezes
- Heart: tachycardia
- Abdomen: NSA
- Skin: no needle marks or cellulitis
Laboratory
- CBC: WBC 11,700 cells/mcL with 80% polymorphonuclear leukocytes, otherwise normal
- Basic metabolic panel: normal
- Brain natriuretic peptide: 120 pg/ml
- Urine drug screen was negative for cocaine but positive for marijuana.
- D-dimer: 0.32 mcg/mL
Hospital Course
He was admitted to the ICU but quickly deteriorated and was intubated for hypoxemia. Empiric ceftriaxone and levofloxacin were begun.
Chest x-ray demonstrated bilateral patchy airspace opacities (Figure 1).

Figure 1. Admission chest x-ray.
Which of the following should be done next? (click on the correct answer to proceed to the second of six pages)
- Bedside cardiac ultrasound
- Coccidioidomycosis serology
- CT scan of the chest
- 1 and 3
- All of the above
Cite as: Assar S, Singarajah CU. January 2017 critical care case of the month. Southwest J Pulm Crit Care. 2017;14(1):6-13. doi: https://doi.org/10.13175/swjpcc143-16 PDF
December 2016 Critical Care Case of the Month
Theodore Loftsgard APRN, ACNP
Department of Anesthesiology
Mayo Clinic Minnesota
Rochester, MN USA
Critical Care Case of the Month CME Information
Members of the Arizona, New Mexico, Colorado and California Thoracic Societies and the Mayo Clinic are able to receive 0.25 AMA PRA Category 1 Credits™ for each case they complete. Completion of an evaluation form is required to receive credit and a link is provided on the last panel of the activity.
0.25 AMA PRA Category 1 Credit(s)™
Estimated time to complete this activity: 0.25 hours
Lead Author(s): Theodore Loftsgard APRN, ACNP. All Faculty, CME Planning Committee Members, and the CME Office Reviewers have disclosed that they do not have any relevant financial relationships with commercial interests that would constitute a conflict of interest concerning this CME activity.
Learning Objectives:
As a result of this activity I will be better able to:
- Correctly interpret and identify clinical practices supported by the highest quality available evidence.
- Will be better able to establsh the optimal evaluation leading to a correct diagnosis for patients with pulmonary, critical care and sleep disorders.
- Will improve the translation of the most current clinical information into the delivery of high quality care for patients.
- Will integrate new treatment options in discussing available treatment alternatives for patients with pulmonary, critical care and sleep related disorders.
Learning Format: Case-based, interactive online course, including mandatory assessment questions (number of questions varies by case). Please also read the Technical Requirements.
CME Sponsor: University of Arizona College of Medicine
Current Approval Period: January 1, 2015-December 31, 2016
Financial Support Received: None
History of Present Illness
A 62-year-old lady with primary biliary cirrhosis/autoimmune hepatitis listed for liver transplantation was admitted to the general medicine floor with progressive lethargy. She had progressive fatigue for about 10 days prior to admission. She had not been able to walk for the last few days; had anorexia; had not had a bowel movement for approximately one week; and had not taken her medicines for 4 days according to her daughter. Her family was concerned with her progressive lethargy; her darkening urine; and progressive jaundice.
She had been managed for several years on mycophenolate mofetil, budesonide, and ursodiol. She had increasing problems with ascites and had paracentesis performed about every 4 days despite taking Lasix and spironolactone. She had early encephalopathy manifested by increasing problems with word finding but had not received lactulose.
Past Medical History
She has a history of esophageal varices, recurrent cellulitis and obesity.
Physical Examination
Vital Signs: P 121 beats/min, BP 102/35 mm Hg, T 37.5◦ C, R 25 breaths/min
General: She was lethargic, somewhat confused but oriented to time, place and person.
Lungs: shallow respirations.
Heart: regular rhythm with a tachycardia.
Abdomen: distended with a fluid wave.
Radiography
Portable chest and abdominal x-rays were performed (Figure 1).


Figure 1. Admission chest (A) and abdominal (B) radiographs.
Which of the following best describes the x-rays? (Click on the correct answer to proceed to the second of six pages)
- The abdominal x-ray shows diffuse, nonspecific gaseous distention
- The abdominal x-ray shows gastrointestinal perforation
- The chest x-ray shows bilateral atelectasis
- The chest x-ray shows bilateral pneumonia
- 1 and 3
- 2 and 4
- All of the above
Cite as: Loftsgard T. December 2016 critical care case of the month. Southwest J Pulm Crit Care. 2016;13(6):278-84. doi: https://doi.org/10.13175/swjpcc104-16 PDF

